Atomically Dispersed Main Group Magnesium on Cadmium
Sulfide as the Active Site for Promoting Photocatalytic Hydrogen Evolution
Catalysis
Ran Chen, Juan Chen, Huinan Che, Gang Zhou, Yanhui Ao* and Bin Liu*
Chin. J. Struct. Chem. 2022, 41, 2201014-2201018 DOI: 10.14102/j.cnki.0254-5861.2021-0027
January 13, 2022
CdS, hydrogen, photocatalysis, atomically dispersed, main group metal
ABSTRACT
Photoabsorption
charge separation/transfer and surface reaction are the three main factors
influencing the efficiency of photocatalysis. Band structure engineering has
been extensively applied to improve the light absorption of photocatalysts,
however, most of the developed photocatalysts still suffer from low
photocatalytic performance due to the limited active site(s) and fast
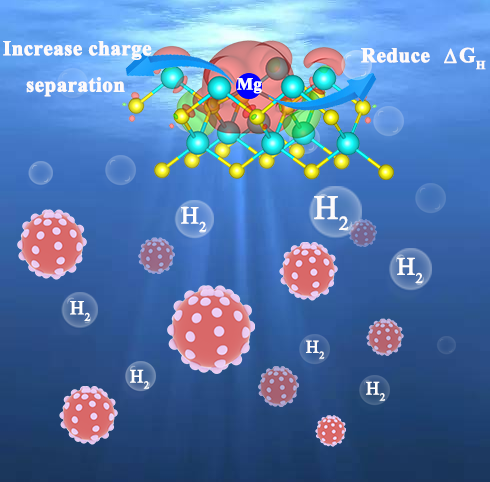
recombination of photogenerated charge carriers. In this work, atomically
dispersed main group magnesium (Mg) is introduced onto CdS monodispersed
nanospheres, which greatly enhances the photocatalytic hydrogen evolution
reaction. The photocatalytic hydrogen evolution reaction rate reaches 30.6
mmol·gcatalyst-1·h-1, which is about 11.8 and
2.5 times that of pure CdS and Pt (2wt.%)-CdS. The atomically dispersed Mg on
CdS acts as an electron sink to trap photogenerated electrons, and at the same time, greatly reduces the Gibbs free energy of hydrogen
evolution reaction (HER) and accelerates HER.