Construction of ZnIn2S4-CdIn2S4 Microspheres for Efficient Photocatalytic Reduction of CO2 with Visible Light
Shitong Han, Bifang Li, Lijuan Huang, Hailing Xi*, Zhengxin Ding* and Jinlin Long*
Chin. J. Struct. Chem. 2022, 41, 2201007-2201013 DOI: 10.14102/j.cnki.0254-5861.2021-0026
January 13, 2022
CO2RR, visible light photocatalysis, heterojunction, CdIn2S4
ABSTRACT
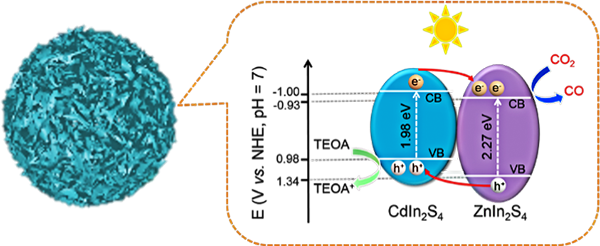
ZnIn2S4 has emerged in water
splitting and degradation of dyes due to its good stability and light
absorption properties. However, there are still few reports of CO2 photoreduction. Herein, we successfully synthesized ZnIn2S4 and obtained a series of ZnIn2S4-CdIn2S4 heterostructured microspheres through the ion exchange method, and first used
them in photocatalytic CO2 reduction in noble-metal-free systems.
The activity results showed that these ZnIn2S4-CdIn2S4 photocatalysts exhibit excellent catalytic activity under visible light,
and the best CO yield is as high as 33.57 μmol·h-1 with a
selectivity of 91%. Furthermore, the stability and reusability of ZnIn2S4-CdIn2S4 was firmly confirmed by diverse characterizations, including X-ray diffraction
(XRD), scanning electron microscopy (SEM), transmission electron microscopy
(TEM), X-ray photoelectron spectroscopy (XPS), energy-dispersive X-ray
spectroscopy (EDX) and N2 adsorption measurements.