Hydrogen spillover enhances the selective hydrogenation of α,β-unsaturated aldehydes on the Cu–O–Ce interface
Jinyuan Cui, Tingting Yang, Teng Xu, Jin Lin, Kunlong Liu*, Pengxin Liu* Submit a Manuscript
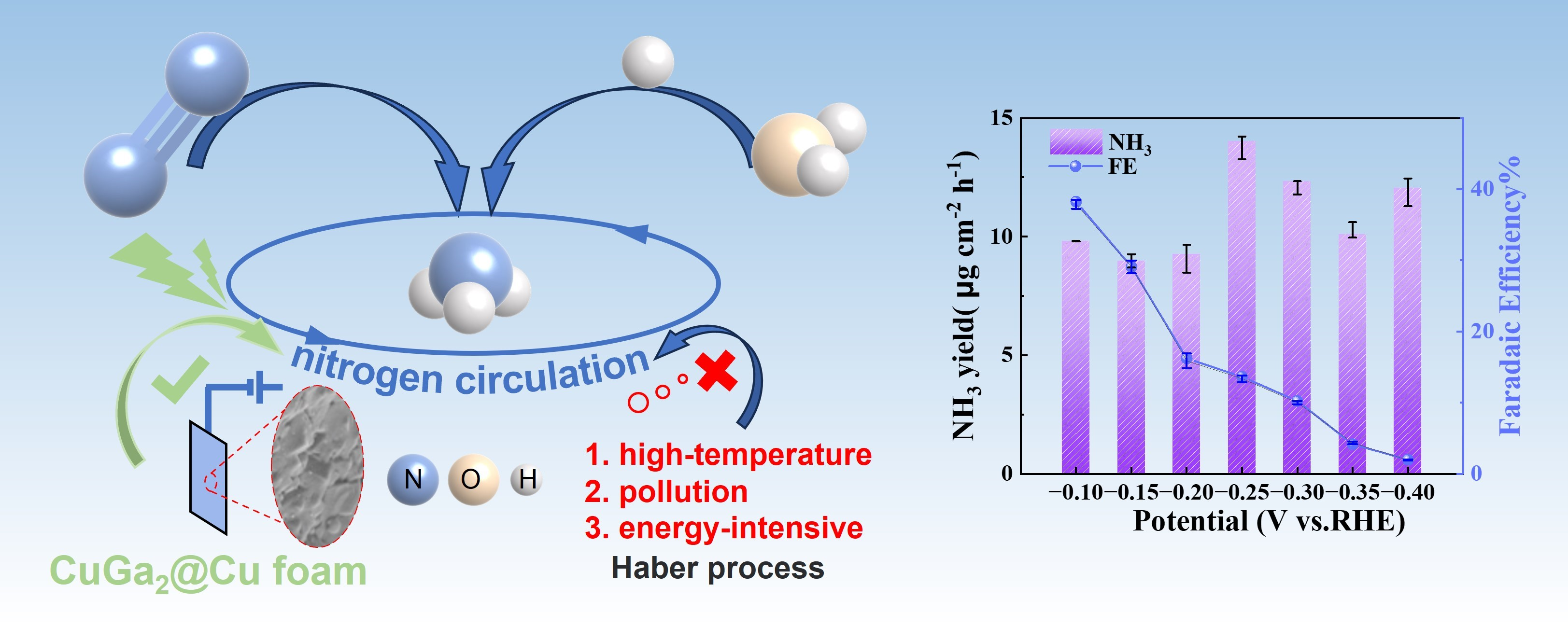
Bin Chen#, Chaoyang Zheng#, Dehuan Shi, Yi Huang, Renxia Deng, Yang Wei, Zheyuan Liu, Yan Yu*, Shenghong Zhong*
Chin. J. Struct. Chem., 2025, 44(1), 100468. DOI: 10.1016/j.cjsc.2024.100468
January 1, 2025
Electrochemical nitrogen fixation; p-d orbital hybridization; Bimetallic catalyst; CuGa2 alloy; Liquid metals
ABSTRACT
In the quest to align with industrial benchmarks, a noteworthy gap remains in the field of electrochemical nitrogen fixation, particularly in achieving high Faradaic efficiency (FE) and yield. The electrocatalytic nitrogen fixation process faces considerable hurdles due to the difficulty of cleaving the highly stable N≡N triple bond. Additionally, the electrochemical pathway for nitrogen fixation is often compromised by the concurrent hydrogen evolution reaction (HER), which competes aggressively for electrons and active sites on the catalyst surface, thereby reducing the FE of nitrogen reduction reactions (NRR). To surmount these challenges, this study introduces an innovative bimetallic catalyst, CuGa2, synthesized through p-d orbital hybridization to selectively facilitate N2 electroreduction. This catalyst has demonstrated a remarkable NH₃ yield of 9.82 μg h-1 cm-2 and an associated Faradaic efficiency of 38.25%. Our findings elucidate that the distinctive p-d hybridization interaction between Ga and Cu enhances NH3 selectivity by reducing the reaction energy barrier for hydrogenation and suppressing hydrogen evolution. This insight highlights the significance of the p-d orbital hybridization in optimizing the electrocatalytic performance of CuGa2 for nitrogen fixation.