Cover Picture
P-Ni4Mo Catalyst for Seawater Electrolysis with High Current Density and Durability
Explicating the Role of Metal Centers in Porphyrin-Based MOFs of PCN-222(M) for Electrochemical Reduction of CO2
Mengjie Liu, Mengting Peng, Baoxia Dong*, Yunlei Teng, Ligang Feng and Qiang Xu*
Chin. J. Struct. Chem. 2022, 41, 2207046-2207052 DOI: 10.14102/j.cnki.0254-5861.2022-0057
July 18, 2022
PCN-222, electrochemical reduction of CO2, DFT calculation, MOFs
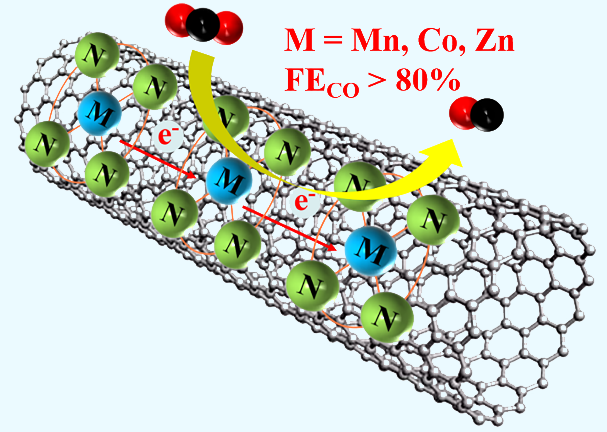
ABSTRACT
The
porphyrin-based MOFs formed by combining Zr6 clusters and porphyrin
carboxylic acids with clear M-N4 active centers show unique
advantages in electrocatalytic reduction of CO2 (CO2RR).
However, its conductivity is still the bottleneck that limits its catalytic
activity due to the electrical insulation of the Zr cluster. Therefore, the porphyrin-based MOFs of PCN-222(M) (M = Mn, Co, Ni, Zn) with
explicit M-N4 coordination were combined with the highly conductive
material carbon nanotube (CNT) for discussing the influence of metal centers on
the CO2RR performance based on
theoretical calculations and experimental observations. The results show that
the PCN-222(Mn)/CNT, PCN-222(Co)/CNT, and
PCN-222(Zn)/CNT all exhibit high selectivity to CO (FECO > 80%) in
the range of -0.60 to -0.70 V vs. RHE. The FECOmax of
PCN-222(Mn)/CNT (-0.60 V vs. RHE), PCN-222(Co)/CNT (-0.65 V vs. RHE), and
PCN-222(Zn)/CNT (-0.70 V vs. RHE) are 88.5%, 89.3% and 92.5%, respectively. The
high catalytic activity of PCN-222(Mn)/CNT and PCN-222(Co)/CNT comes from the
excellent electron mobility of their porphyrin rings and their low ΔG*COOH (0.87 and 0.58 eV). It reveals that the strength of backbonding π of the transition metal and its influence on the
electron mobility in the porphyrin ring can affect its CO2RR
activity.