Cover Picture
P-Ni4Mo Catalyst for Seawater Electrolysis with High Current Density and Durability
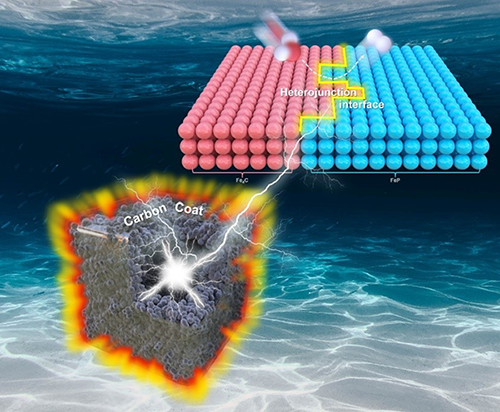
Hollow Fe4C/FeP Nanoboxes with Heterostructure and Carbon Armor for Efficient and Stable Hydrogen Evolution
Jing-Yi Xie, Hui-Ying Zhao, Yi-Wen Dong, Yang Wu, Da-Peng Liu*, Yong-Ming Chai and Bin Dong*
Chin. J. Struct. Chem. 2022, 41, 2207053-2207058 DOI: 10.14102/j.cnki.0254-5861.2022-0102
July 18, 2022
carbon armor, hollow box, heterostructured electrocatalysts, hydrogen evolution reaction
ABSTRACT
The
heterojunction interfacial modulation of FeP is an effective strategy to
regulate the intrinsic activity and stability, which is a major challenge to
promote the industrial application of FeP-based electrocatalysts. Herein,
hollow Fe4C/FeP box with heterojunction interface and carbon armor
is successfully synthesized, which can expose numerous active sites and protect
catalyst from corrosion. Electrochemical measurements show that Fe4C/FeP
exhibits excellent hydrogen evolution activity and stability. It only needs 180
mV to achieve the current density of 10 mA cm-2. The high-activity
may be due to the synergistic effects of porous framework, graphitic carbon
coating and heterojunction structure of Fe4C
and FeP, which optimize the electronic structure and accelerates electron
transfer. In addition, the target catalyst can withstand 5000 cycles of CV
testing without significant change in properties. The
excellent stability may be attributed to the graphitic carbon coating as the
armor that can prevent the catalyst from corrosion of electrolyte. This
work may provide a synthetic approach to produce a series of carbon-coated and
heterojunction structure of transition metal phosphides for water splitting.