Cover Picture
Deep learning-based workflow for atomic image denoising and chemical identification
Ke Ma, Shiqiang Feng, Haihui Hu, Yimeng Cai, Dechao Chen, Lili Han*
Submit a Manuscript
Deep learning-based workflow for atomic image denoising and chemical identification
Ke Ma, Shiqiang Feng, Haihui Hu, Yimeng Cai, Dechao Chen, Lili Han*
Submit a Manuscript
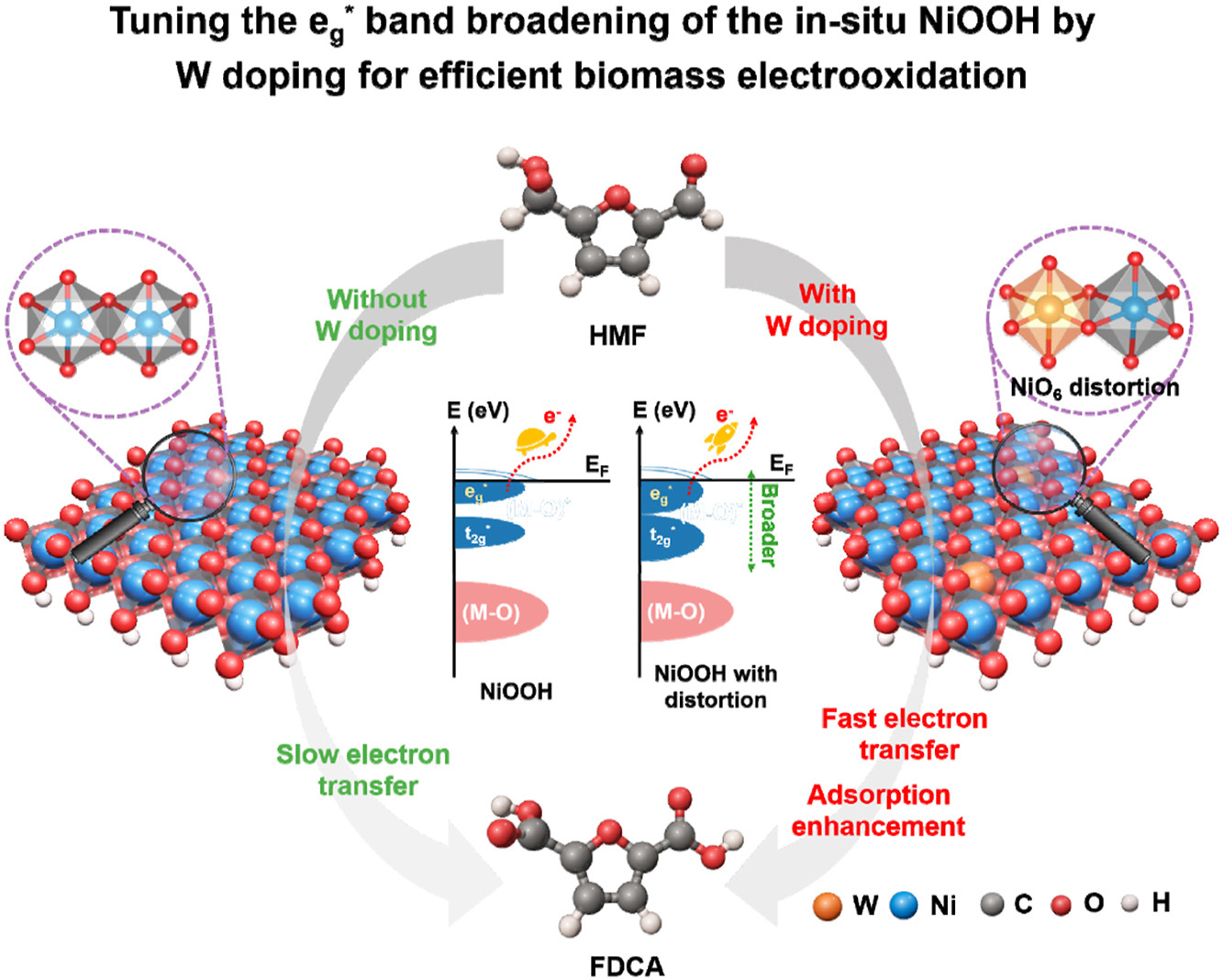
Tuning the eg* band broadening of the in-situ NiOOH by W doping for efficient biomass electrooxidation
Jinlv Wei, Jianlong Zhang, Huan Wen, Zhixiang Zhai, Fangyuan Guan, Zelong Sun, Jia Wu*, Shibin Yin*
Chin. J. Struct. Chem., 2025, 44(5), 100541. DOI: 10.1016/j.cjsc.2025.100541
May 1, 2025
5-Hydroxymethylfurfural oxidation; 2,5-Furandicarboxylic acid; W-doped; Jahn-Teller distortion; NiOOH
ABSTRACT
5-Hydroxymethylfurfural electrooxidation reaction (HMFOR) provides a promising route for producing high-value-added compounds. It is generally believed that NiOOH is the active species in HMFOR process, but its inherently poor electron transfer ability leads to limited catalytic activity. In this work, a W doping strategy is adopted to regulate the electron transfer between NiOOH and reaction molecules. Electrochemical results show that W-doped Ni5P4-R exhibits excellent electrochemical performance for the oxidation of HMF to FDCA with the conversion of HMF and yield of FDCA both close to ∼100%. Density functional theory and in-situ characterization reveal that the introduction of W causes the distortion of NiOOH lattice, resulting in the Jahn-Teller distortion and the elimination of orbital degeneracy, thereby broadening the eg∗ band of NiOOH. This feature is beneficial for the electron transfer between W-doped NiOOH and HMF (1.31 e−), thereby promoting the C–H bond activation of the aldehyde group in HMF and effectively reducing the energy barrier of rate-determining step (RDS).