Cover Picture
P-Ni4Mo Catalyst for Seawater Electrolysis with High Current Density and Durability
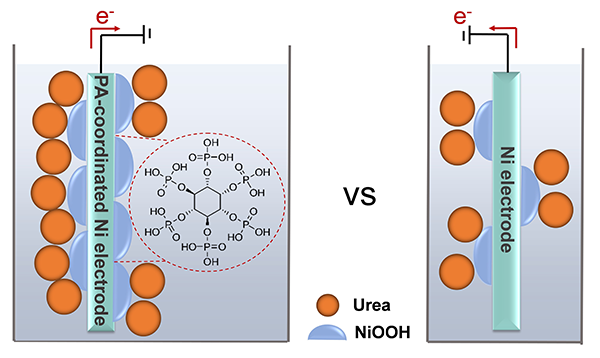
Phytate-Coordination Triggered Enrichment of Surface NiOOH Species on Nickel Foam for Efficient Urea Electrooxidation
Jiayuan Li*, Yuefei Li, Qingyu Xue, Yuchi Gao and Yuanyuan Ma
Chin. J. Struct. Chem. 2022, 41, 2207035-2207039 DOI: 10.14102/j.cnki.0254-5861.2022-0095
July 18, 2022
phytates, surface coordination, urea electrooxidation reaction, electrocatalysis
ABSTRACT
Nickel (Ni)-based materials are promising electrocatalysts for the urea
electrooxidation reaction, as the in situ formed NiOOH species on their surface during operation are catalytically active
sites. In this work, phytate-coordinated Ni foam (PA-NF) is shown to deliver a
high catalytic performance toward the urea electrooxidation reaction, with a low
potential of 1.38 V at 10 mA/cm2, a low Tafel slope of 64.1 mV/dec,
and superior catalytic stability; a performance comparable to state-of-the-art Ni-based catalysts. Electrochemical
characterization alongside the control experiments revealed that such a high performance could be
ascribed to kinetically-accelerated surface reconstruction and the enrichment
of NiOOH active species on the PA-NF surface during the electrooxidation of urea
owing to PA-coordination induced upshift of d-band center of Ni sites. Overall, a new strategy is provided for the
design of an efficient and universal Ni-based catalyst for the electrooxidation
of urea, which can also be extended to other transition-metal-based systems.