Ni(OH)2 Derived from NiS2 Induced by Reflux Playing Three Roles for Hydrogen/oxygen Evolution Reaction
Sheng-Jun Xu*, Ya-Nan Zhou, Guo-Ping Shen and Bin Dong* Submit a Manuscript
Qianqian Tang, Longshuai Zhang* and Jian-Ping Zou*
Chin. J. Struct. Chem. 2022, 41, 2208001-2208002 DOI: 10.14102/j.cnki.0254-5861.2022-0129
July 25, 2022
ABSTRACT
As promising approaches in wastewater treatment, advanced oxidation processes (AOPs) can efficiently degrade and mineralize the organic wastes and contaminants by generating reactive radicals like ·OH, ·SO4-, and ·O2-. However, due to the stronger oxidability, these reactive radicals exhibit lower selectivity of degradation. Conversely, 1O2, which has high selectivity for electron-rich substances degradation by removing electrons, is a weak oxidant with long life and wide pH tolerance. Currently, the commonly used method of producing 1O2 is the disproportionation of O2-. But the competitive reaction, the Haber-Weiss reaction (·O2- + H2O2 → ·OH + OH- + O2), significantly reduces the yield of 1O2. Thus, to activate PMS or PDS by effective catalysts for highly selective and efficient generation of 1O2 is necessary. Owing to the atomic dispersion of active sites and higher selectivity, single-atom catalysts (SACs) have great potential to approach that goal. Particularly, the N-doped carbon supported SACs can efficiently activate PMS with markedly improved 1O2 generation selectivity. However, the sparse and random distribution of N atoms which act as coordination sites for metal atoms causes the low metal loading and inhomogeneous local coordination environments, and further retards the high-efficient generation of 1O2.
Graphitic carbon nitride (g-C3N4), which has abundant and uniform N sites as single metal atomic fixation sites, has been considered as ideal support to obtain SACs with uniform and densely loaded atomic dispersion of metal active sites. Nevertheless, the current g-C3N4 supported SACs are generally accompanied by metal clusters or even nanoparticles inevitably due to the migration of metal ions during the pyrolysis process, especially in the case of high metal content. Designing an appropriate strategy to prepare high-loading g-C3N4 supported SACs with uniform single metal atom sites is an effective approach to facilitate the efficient activation of PMS to generate 1O2 with high selectivity.[1] Recently, writing in Angewandte Chemie International Edition, Zou and colleagues reported that carbon nitride supported high-loading Fe SAC (Fe1/CN) can efficiently activate PMS to generate 1O2 with 100% selectivity.
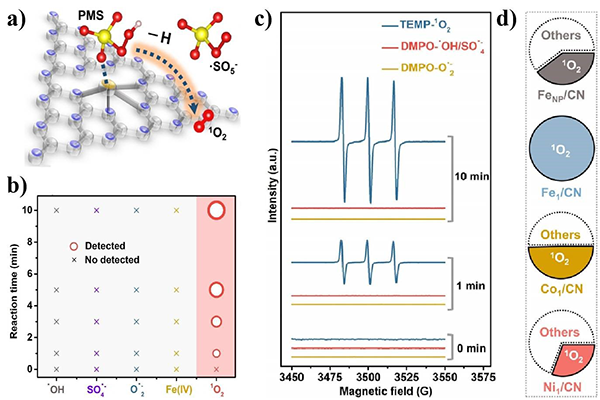
The Fe1/CN catalyst with a loading up to 11.2 wt% is prepared via the supermolecule method.[2] The Fe1/CN catalyst is proved to have atomic dispersion of Fe sites with highly uniform Fe-N4 centers. As a result, compared to CN supported Fe nanoparticles (FeNP/CN) catalysts, Fe1/CN can efficiently activate PMS to generate 1O2 with 100% selectivity (Figure 1a). The sole generation of 1O2 was proved by radical quenching experiments and electron paramagnetic resonance (EPR) tests (Figure 1b-d). The high efficiency and selective generation of 1O2 from Fe1/CN activated PMS can rapidly degrade and mineralize p-chlorophenol (4-CP) with higher apparent rate constants (Kobs) even more than the homogeneous Fe2+ in the PMS system did. Due to the sole generation of 1O2, the Fe1/CN activated PMS system displays stronger resistance to obstruction from natural water, and has wider pH tolerance and better cycle stability. The density functional theory (DFT) calculation results demonstrate that the Fe sites in Fe1/CN tend to adsorb the terminal O of PMS, which can facilitate the oxidization of PMS to form ·SO5-, and thereafter efficiently generate 1O2 with 100% selectivity.