Ni(OH)2 Derived from NiS2 Induced by Reflux Playing Three Roles for Hydrogen/oxygen Evolution Reaction
Sheng-Jun Xu*, Ya-Nan Zhou, Guo-Ping Shen and Bin Dong* Submit a Manuscript
-
News & ViewsHigh-Loading Fe Single-Atom Catalyst for 100% Selectivity of 1O2 Generation
Qianqian Tang, Longshuai Zhang* and Jian-Ping Zou*
Chin. J. Struct. Chem. 2022, 41, 2208001-2208002 DOI: 10.14102/j.cnki.0254-5861.2022-0129
July 25, 2022
ABSTRACT
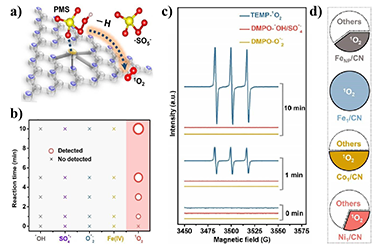
Advanced oxidation processes (AOPs), which generate reactive radicals like ·OH, ·SO4- and ·O2- for degradation and mineralization of organic wastes and contaminants, have been recognized as promising approaches in wastewater treatment. However, due to the stronger oxidability, these reactive radicals exhibit lower selectivity of degradation. Conversely, 1O2 is a weak oxidant that has strong electrophilicity and selectivity to remove electrons from electron-rich substances with long life, wide pH tolerance, and high selectivity. Currently, the commonly used method of producing 1O2 is the disproportionation of O2-. But the competitive reaction, the Haber-Weiss reaction (·O2- + H2O2 → ·OH + OH- + O2), significantly reduces the yield of 1O2. Thus, to activate PMS or PDS by effective catalysts for highly selective and efficient generation of 1O2 is necessary. Single-atom catalysts (SACs) with higher selectivity and efficiency in catalytic reactions are promising catalysts to approach that goal. In particular, the N-doped carbon supported SACs have exhibited significantly enhanced 1O2 generation selectivity from the activated PMS. However, the sparse and random distribution of N atoms, which act as coordination sites for metal atoms, causes the low metal loading and inhomogeneous local coordination environments, and further retards the high-efficient generation of 1O2.
-
CommunicationStrong High Entropy Alloy-Support Interaction Enables Efficient Electrocatalytic Water Splitting at High Current Density
Hongdong Li, Yue Pan, Jianping Lai*, Lei Wang* and Shouhua Feng
Chin. J. Struct. Chem. 2022, 41, 2208003-2208011 DOI: 10.14102/j.cnki.0254-5861.2022-0125
July 25, 2022
high entropy alloy, metal-support interactions, microwave heating, water splitting, activity and durability
ABSTRACT
In electrocatalysis, the stability issue between catalyst and support still needs great attention. Here, a series of high- entropy alloy nanoparticles (HEA-NPs) embedded in carbon cloth (CC) were synthesized by using the scalable strategy-microwave heating. Among them, PtRhCoNiCu/CC exhibits outstanding hydrogen evolution reaction (HER) activity (19 and 170 mV overpotential at 10 and 1000 mA cm-2) and stability (150 h), outperforming other recently reported HEAs catalysts. IrRuCoNiCu/CC displays superior oxygen evolution reaction (OER) activity (166 and 354 mV overpotential at 10 and 1000 mA cm-2) and stability (150 h), and shows a lower overpotential than recently reported HEA catalysts. In water splitting, IrRuCoNiCu/CC(+)//PtRhCoNiCu/CC(-) electrolyzer achieves 500 mA cm-2 (1000 mA cm-2) high current density at 1.76 V (1.88 V) and exhibits excellent stability, which is one of the best catalysts currently. Therefore, the novel supported HEA catalyst with high stability is expected to be a promising candidate material for industrialized water splitting. -
Communication
Sulfonate-Functionalized Polyoxovanadate-Based Metal-Organic Polyhedra for Enhanced Proton Conduction via the Synergy of Linker and Metal Cluster Vertex
Yu Zhang, Shan-Shan Liu, Bo Li, Hanqi You, Longxi Zhang, Zhenyi Zhang, Hong-Ying Zang*, Qi Zheng* and Weimin Xuan*
Chin. J. Struct. Chem. 2022, 41, 2208012-2208017 DOI: 10.14102/j.cnki.0254-5861.2022-0127
July 25, 2022
metal-organic polyhedra, proton conduction, polyoxovanadate, synergistic effect
ABSTRACT
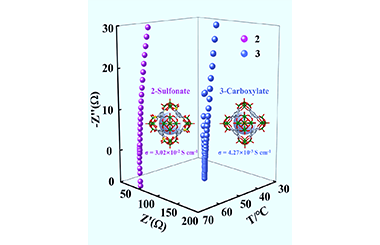
Metal-organic polyhedra (MOPs) have emerged as novel porous platforms for proton conduction, however, the concerted employment of both linker and metal cluster vertex is rarely applied for the fabrication of MOPs-based high conducting materials. Herein we report the synthesis of sulfonate-functionalized polyoxovanadate-based MOPs for enhanced proton conduction via the synergistic effect from linker and metal cluster node. MOPs 1 and 2 exhibit octahedral cage configuration constructed from {V5O9Cl} vertex and 5-sulfoisophthalate linker. Owing to the ordered packing of octahedral cages along three axes, 3D interpenetrated open channels that are lined with high-density sulfonates are thus formed within 2. Coupled with the proton-conductive {V5O9Cl} vertexs as well as protonated counterions, an extensive H-bonded network is therefore generated for facile proton transfer. 2 exhibits high proton conductivity of 3.02×10-2 S cm-1 at 65 °C under 90% RH, recording the highest value for MOPs pellet sample. This value is enhanced ~1 order of magnitude compared with that of carboxylate-functionalized analogue 3, clearly illustrating the advantage of combining linker and metal cluster node for enhanced proton conduction. This work will further promote the exploitation of high proton conductive MOPs-based materials by the synergy design strategy. -
ArticleGraphene-Coated 1D MoTe2 Nanorods as Anode for Enhancing Lithium-Ion Battery Performance
Shuwei Chai, Xiong Xiao, Yabei Li and Changhua An*
Chin. J. Struct. Chem. 2022, 41, 2208018-2208024 DOI: 10.14102/j.cnki.0254-5861.2022-0050
July 25, 2022
MoTe2, nanorods, graphene, lithium ion battery
ABSTRACT
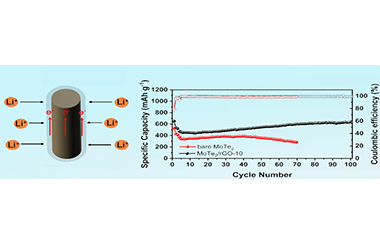
One-dimensional nanostructures (1D) with short ion diffusion distance and fast ion transport path are excellent for lithium-ion batteries (LIBs). However, the nature of layered transition metal dichalcogenides makes it difficult to form 1D nanohybrids. Here, the MoTe2 nanorods with an average diameter of 100-200 nm and length of 1-3 μm encapsulated by reduced graphene oxide (MoTe2/rGO) have been fabricated via in-situ reaction of GO coated Mo3O10(C2H10N2) nanowires with Te under Ar/H2 atmosphere. When applied as anode of LIBs, the MoTe2/rGO delivers a high reversible capacity (637 mA h g-1 after 100 cycles at 0.2 A g-1), good rate capability (374 mA h g-1 at 2 A g-1) and excellent stability (360 mA h g-1 after 200 cycles at 0.5 A g-1), which surpasses bare MoTe2 nanorods and bulk MoTe2 crystallite. Furthermore, a lithium-ion full cell constructed by coupling MoTe2/rGO anode and LiCoO2 cathode shows a capacity of 105 mA h g-1 at 0.1 C. The enhanced performance mainly benefits from the advantages of 1D nanostructure, and meanwhile the rGO thin layers are able to improve the conductivity and maintain the structural stability. This work provides a simple pathway for the synthesis of 1D TMDs nanostructures for energy storage and conversion. -
ArticleWO3@Fe2O3 Core-Shell Heterojunction Photoanodes for Efficient Photoelectrochemical Water Splitting
Guobing Mao, Heng Wu, Tianyang Qiu, Dingjie Bao, Longjie Lai, Wenguang Tu* and Qi Liu*
Chin. J. Struct. Chem. 2022, 41, 2208025-2208030 DOI: 10.14102/j.cnki.0254-5861.2022-0086
July 25, 2022
host/guest photoelectrodes, WO3, a-Fe2O3, core-shell nanostructures, one-dimensional nanoarray
ABSTRACT
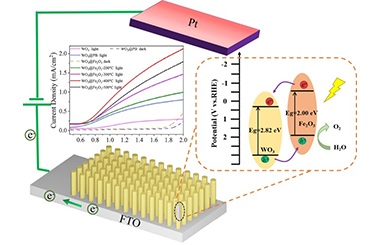
Photoelectrochemical (PEC) hydrogen production from water splitting is a green technology to convert solar energy into renewable hydrogen fuel. The construction of host/guest architecture in semiconductor photoanodes has been proven to be an effective strategy to improve solar-to-fuel conversion efficiency. In this study, WO3@Fe2O3 core-shell nanoarray heterojunction photoanodes are synthesized from the in-situ decomposition of WO3@Prussian blue (WO3@PB) and then used as host/guest photoanodes for photoelectrochemical water splitting, during which Fe2O3 serves as guest material to absorb visible solar light and WO3 can act as host scaffolds to collect electrons at the contact. The prepared WO3@Fe2O3 shows the enhanced photocurrent density of 1.26 mA cm-2 (under visible light) at 1.23 V vs RHE and a superior IPEC of 24.4% at 350 nm, which is higher than that of WO3@PB and pure WO3 (0.43 mA/cm-2 and 16.3%, 0.18 mA/cm-2 and 11.5%) respectively, owing to the efficient light-harvesting from Fe2O3 and the enhanced electron-hole pairs separation from the formation of type-II heterojunctions, and the direct and ordered charge transport channels from the one-dimensional (1D) WO3 nanoarray nanostructures. Therefore, this work provides an alternative insight into the construction of sustainable and cost-effective photoanodes to enhance the efficiency of the solar-driven water splitting. -
ArticleNi3N Modified MOF Heterostructure with Tailored Electronic Structure for Efficient Overall Water Splitting
Hongnan Jia, Na Yao, Juan Zhu, Yujia Liu, Yunhao Lao, Hengjiang Cong* and Wei Luo*
Chin. J. Struct. Chem. 2022, 41, 2208031-2208036 DOI: 10.14102/j.cnki.0254-5861.2022-0112
July 25, 2022
hydrogen evolution reaction, Ni3N, MOF, DFT calculation, water splitting
ABSTRACT
Exploring bifunctional electrocatalysts with high-efficiency and stability toward overall water splitting is desirable for sustainable energy technologies, yet challenging. Herein, we report the construction of Ni3N on the surface of Ni-MOF-74 through an in-situ nitriding process. The obtained Ni-MOF-74/Ni3N exhibits remarkable HER activity, with an overpotential of 73 mV to deliver 10 mA cm−2. Theoretical calculations and experimental study demonstrate the electron transport between Ni3N and Ni-MOF-74, leading to the improved H2O adsorption, optimized hydrogen adsorption, and increased Had diffusion, which contributes to the enhanced HER performance. Besides, the obtained Ni-MOF-74/Ni3N also possesses outstanding activity toward OER and overall water splitting. -
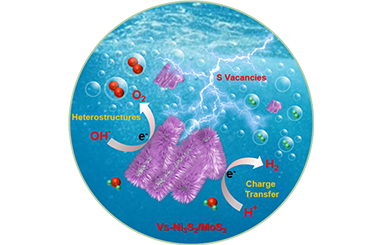
ArticleCo-Construction of Sulfur Vacancies and Heterogeneous Interface into Ni3S2/MoS2 Catalysts to Achieve Highly Efficient Overall Water Splitting
Zhaoyang An, Hui Xue*, Jing Sun, Niankun Guo, Tianshan Song, Jiawen Sun, Yi-Ru Hao and Qin Wang*
Chin. J. Struct. Chem. 2022, 41, 2208037-2208043 DOI: 10.14102/j.cnki.0254-5861.2022-0130
July 25, 2022
sulfur vacancies, heterostructures, transition metal chalcogenides, overall water splitting
ABSTRACT
Integrating the advantages of anion vacancies and heterostructures into the catalytic materials may increase the binding affinities to intermediates, provide more active sites, and significantly promote the activity of overall water splitting. However, the successful assembly of anion vacancies and heterostructures for high-efficiency water splitting performance is still challenging. In this work, we ingeniously present the co-construction of sulfur vacancies and heterogeneous interface into Ni3S2/MoS2 catalysts on nickel foam (NF). The introduction of sulfur vacancies and Ni3S2/MoS2 heterostructures can significantly improve electron and ion transport, effectively improve structural stability, and enhance overall water splitting activity. The obtained VS-Ni3S2/MoS2 catalysts (VS stands for sulfur vacancies) exhibit superior OER and HER activities, and the overpotentials for OER and HER are 180 and 71 mV at 10 mA·cm-2, respectively. Furthermore, a low water splitting voltage of 1.46 V is required at 10 mA·cm-2 for the VS-Ni3S2/MoS2 catalysts, which is considerably lower than most of the water splitting electrocatalysts currently reported. This work offers an effective mean for the preparation of catalysts with both anion vacancies and heterostructures for achieving high-performance alkaline overall water splitting. -
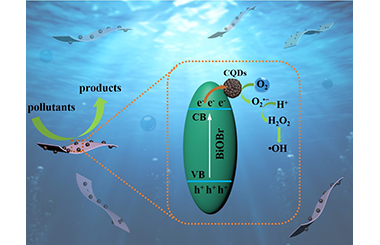
ArticleIn-situ Synthesis of CQDs/BiOBr Material via Mechanical Ball Milling with Enhanced Photocatalytic Performances
Xingwang Yan, Bin Wang, Mengxia Ji, Qi Jiang, Gaopeng Liu, Pengjun Liu*, Sheng Yin, Huaming Li and Jiexiang Xia*
Chin. J. Struct. Chem. 2022, 41, 2208044-2208051 DOI: 10.14102/j.cnki.0254-5861.2022-0141
July 25, 2022
CQDs, BiOBr, photocatalysis, pollutant degradation
ABSTRACT
Designing simple, efficient, and environmentally friendly methods to construct high-efficient photocatalysts is an important strategy to promote the further development of the field of photocatalysis. Herein, flower-like carbon quantum dots (CQDs)/BiOBr composite photocatalysts have been prepared via in-situ synthesis by mechanical ball milling in the existence of ionic liquid. The CQDs/BiOBr composites exhibit higher photo-degradation performance for tetracycline (TC) than BiOBr monomer and the commercial Bi2O3 under visible light irradiation. For comparison, the different Br sources and synthetic methods are chosen to prepare BiOBr and CQDs/BiOBr composites. Photocatalysts prepared by ball milling and ionic liquid present significantly enhanced photocatalytic performance for removing TC. In addition, the introduction of CQDs could distinctly enhance the photocatalytic performances of pure BiOBr. The reason is that CQDs as electron acceptor effectively separate electrons and holes and inhibit their recombination. The intermediates during photocatalytic degradation were tested using liquid chromatography-mass spectrometry (LC-MS) and possible degradation pathways were given. During degradation, •OH, O2•- and h+ were identified to be the main active species based on electron spin resonance (ESR) spectra and free radical trapping experiments. A possible mechanism of CQDs/BiOBr with enhanced photocatalytic performances was further proposed.
-
Article
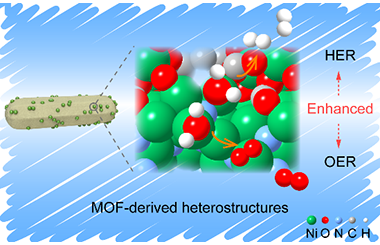
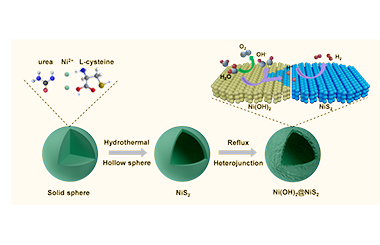
Ni(OH)2 Derived from NiS2 Induced by Reflux Playing Three Roles for Hydrogen/oxygen Evolution Reaction
Sheng-Jun Xu*, Ya-Nan Zhou, Guo-Ping Shen and Bin Dong*
Chin. J. Struct. Chem. 2022, 41, 2208052-2208057 DOI: 10.14102/j.cnki.0254-5861.2022-0143
July 25, 2022
Ni(OH)2, NiS2, heterostructures, oxygen evolution reaction, hydrogen evolution reaction
ABSTRACT
Developing efficient and promising non-noble catalysts for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is vital but still a huge challenge for the clean energy system. Herein, we have integrated the active components for OER (Ni(OH)2) and HER (NiS2 and Ni(OH)2) into Ni(OH)2@NiS2 heterostructures by a facile reflux method. The in-situ formed Ni(OH)2 thin layer is coated on the surface of hollow NiS2 nanosphere. The uniform Ni(OH)2@NiS2 hollow sphere processes enlarge the electrochemically active specific surface area and enhance the intrinsic activity compared to NiS2 precursor, which affords a current density of 10 mA cm-2 at the overpotential of 309 mV and 100 mA cm-2 at 359 mV for OER. Meanwhile, Ni(OH)2@NiS2 can reach 10 mA cm-2 at 233 mV for HER, superior to pure NiS2. The enhanced performance can be attributed to the synergy between Ni(OH)2 and NiS2. Specifically, Ni(OH)2 has three functions for water splitting: providing active sites for hydrogen adsorption and hydroxyl group desorption and working as real OER active sites. Moreover, Ni(OH)2@NiS2 displays great stability for OER (50 h) and HER (30 h).
-
Article
Synergism of 1D CdS/2D Modified Ti3C2Tx MXene Heterojunctions for Boosted Photocatalytic Hydrogen Production
Shi Cheng, Qianqian Xiong, Chengxiao Zhao* and Xiaofei Yang*
Chin. J. Struct. Chem. 2022, 41, 2208058-2208064 DOI: 10.14102/j.cnki.0254-5861.2022-0151
July 25, 2022
CdS, MXene, heterojunction, water splitting, hydrogen evolution reaction
ABSTRACT
Rational design and controllable synthesis of visible-light-responsive photocatalysts that exhibit both good hydrogen-producing efficiency and stability in the water splitting reaction are undoubtedly a challenge. Here we report an integrated CdS nanorod/oxygen-terminated Ti3C2Tx MXene nanosheet heterojunction with a high catalytic hydrogen evolution reaction (HER) activity. By incorporating one-dimensional (1D) CdS nanorods onto annealed ultrathin two-dimensional (2D) MXene nanoshees, the mixed-dimensional 1D/2D heterojunction achieved a hydrogen-evolving rate of 8.87 mmol×g-1×h-1, much higher than that of bulk CdS and CdS/unmodified MXene hybrid catalysts. The enhanced HER activity and stability of the designed heterojunction catalyst are attributed to the presence of oxygen-containing terminal groups on the surface of thermally treated Ti3C2Tx MXene, extended light absorption spectra as well as the precisely-constructed intimate Schottky contact, implying an accelerated interfacial charge transfer and efficient, long-term photocatalytic hydrogen production performance. The results demonstrate that oxygen-terminated 2D MXene can be well utilized as a functional platform for the development of novel heterojunctioned photocatalysts.