Cover Picture
Two Bisligand-Coordinated Luminescent Zn(II)-Coordination Polymers for Sensing of Ions and Pesticides in Aqueous Solutions
The demon mirror (Zhaoyaojing in Chinese) in the Journey to the West, a Chinese classical literature, can reflect the original shape of demons. Both as-prepared coordination polymers, [Zn(PTA)(DTP)(H2O)2]·(DMF) (CP-1) and [Zn(BTC)(DTP)]·(CH3CN)1.5·(H2O)4 (CP-2), can act as demon mirror to detect the different environmental pollutants. This image presented the magic demon mirror to reflect the presence of pollutants, the demons in environment, which provided an opportunity for the readers to appreciate Chinese traditional culture.
September 2022Vol. 41, Issue 9
-
ReviewStudies on the Luminescence Property of Yttrium-Based Metallo-Fullerenes
Jiayi Liang, Chunru Wang and Taishan Wang*
Chin. J. Struct. Chem. 2022, 41, 2209001-2209007 DOI: 10.14102/j.cnki.0254-5861.2022-0105
September 22, 2022
metallofullerenes, yttrium, luminescence, thermally activated delayed fluorescence, phosphorescence
ABSTRACT
Endohedral metallofullerenes (EMFs) exhibit various properties due to their multiple combinations between internal metals and outer carbon cages. Among them, yttrium-based metallofullerenes have attracted much attention due to their luminescence properties. For example, Y3N@C80 is distinguished by its photoluminescence (PL) properties with a small energy gap between the lowest singlet states (S1) and the triplet excited states (T1) in Y3N@C80, allowing reverse intersystem crossing (RISC) of T1→S1 and resulting in thermally activated delayed fluorescence (TADF). In addition, the PL intensity, lifetime, and quantum yield (QY) of Y3N@C80 all depend on the molecular structure and surrounding environment. Typically, modulation of the PL properties can be achieved by replacing the yttrium metal inside the carbon cage as well as by modifying the carbon cage externally. Here, we focus on the luminescence properties of yttrium-based metallofullerenes, summarize recent research advances, and predict their future development.
-
Review
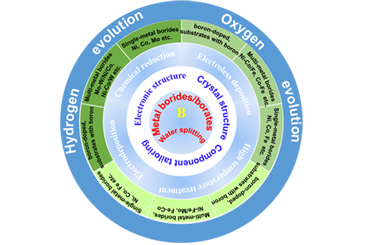
Transition Metal Boride-Based Materials for Electrocatalytic Water Splitting
Feng-Ge Wang, Xin Liu, Qian-Xi Lv, Bin Liu*, Yong-Ming Chai and Bin Dong*
Chin. J. Struct. Chem. 2022, 41, 2209008-2209044 DOI: 10.14102/j.cnki.0254-5861.2022-0117
September 22, 2022
transition metal borides/borates, electrocatalysts, hydrogen evolution reaction (HER), oxygen evolution reaction (OER), overall water splitting
ABSTRACT
Electrocatalytic water splitting to produce hydrogen is an eco-friendly way to achieve sustainable utilization of renewable energy. The industrial application of water electrolysis, which is severely limited by slow kinetic reactions on electrode surfaces, requires the development of highly reactive, low-cost and stable electrocatalytic materials. Transition metal borides/borates have recently emerged as promising electrocatalytic materials for catalyzing hydrogen/oxygen evolution reactions (HER/OER) in inexpensive electrolyzers. However, so far, there has been little comprehensive summary of transition metal borides/borates. Here, this review provides the latest research progress on transition metal borides/borates for electrocatalytic water splitting. The structural characteristics of transition metal borides/borates and their synthesis methods in recent years are discussed. Then, the theoretical and experimental progress of transition metal borides including single-metal borides, multi-metal borides, borate derived and other nanocomposites containing boron (boron-doped nanocomposites/substrate with boron) in electrocatalytic reaction and the role of boron in regulating electrocatalytic performance are further emphasized. Finally, the potential challenges and future prospects of transition metal borides/borates in electrocatalysis are presented. -
Review
The Multiple Modification Road of Li-Rich Manganese-Based Cathode Materials
Yuming Liu, Jingyi Li, Feixiang Wu, Yunjiao Li, Junchao Zheng and Zhenjiang He*
Chin. J. Struct. Chem. 2022, 41, 2209045-2209055 DOI: 10.14102/j.cnki.0254-5861.2022-0170
September 22, 2022
lithium-ion batteries, Li-rich manganese-based, high energy density, multiple modification
ABSTRACT
Li-rich manganese-based cathode materials (LR) are considered as excellent cathode materials for a new generation of lithium-ion batteries causes their outstanding electrochemical performance, friendly price, and environmental friendliness. But defects such as rapid voltage decay and loss of lattice oxygen limit their applications. The electrochemical performance of LR has to be improved by means of modification. The previous single modification methods like element doping, surface coating, structure design, etc. can only optimize the electrochemical performance of LR from one aspect. Recently, multiple modifications, which can combine the advantages of multiple modifications, have been favored by researchers. Here, we comprehensively review the recent progress of multiple modification of LR based on the combination of different modification means. The review and summary of the multiple modification of LR will play a guiding role in its development in the future. -
Review
Recent Progress in π-Conjugated Polymer-Inorganic Heterostructures for Photocatalysis
Yu-Qin Xing and Shi-Yong Liu*
Chin. J. Struct. Chem. 2022, 41, 2209056-2209068 DOI: 10.14102/j.cnki.0254-5861.2022-0188
September 22, 2022
polymer-inorganic composite, heterojunction, photocatalytic water splitting, photocatalytic CO2 reduction, photocatalytic environmental remediation
ABSTRACT
Polymer-inorganic (P-I) soft-hard heterostructures & heterojunction photocatalysts, featured by large interfacial contact, efficient charge separation, broad light absorption and maximized redox capacity, have received great attention for their applications in environmental remediation and energy conversion. In this minireview, the classification and mechanism of P-I heterojunctions, i.e., type-I/II, p-n, Z-scheme and S-scheme heterojunctions, and their preparation methods are firstly introduced. Next, the photocatalytic applications of P-I heterojunctions, including water splitting, environmental remediation and carbon dioxide reduction, are extensively reviewed. Lastly, a brief summary and perspectives on ongoing challenges and opportunities to construct high performance P-I soft-hard photocatalysts are intensively highlighted. We envision this review will provide a picture of the state-of-the-art achievements and promote the photocatalytic applications of P-I heterostructures in energy conversion and environmental remediation. -
Letter
Revealing the Role of Elementary Doping in Photocatalytic Phenol Mineralization
Houkui Xiang, Zhijian Wang and Jiazang Chen*
Chin. J. Struct. Chem. 2022, 41, 2209069-2209073 DOI: 10.14102/j.cnki.0254-5861.2022-0097
September 22, 2022
hole extraction, interfacial electron transfer, hydroxyl radical, elementary doping, photocatalytic mineralization
ABSTRACT
Photocatalytic mineralization of recalcitrant contaminants like phenol in wastewater requires abundant hydroxyl radicals (·OH) to initiate the reaction prior to the ring-opening. We here increase the free energy for adsorption of O* species on TiO2 surface and slightly downshift the band position by tin doping. This can simultaneously promote the generation and suppress the annihilation of ·OH. Besides, tin doping can also facilitate semiconductor-cocatalyst-solution (SCS) interfacial electron transfer by lowering the potential barrier and synergistically enhance the photon utilization. By filming the photocatalyst onto our developed fixed bed reactors, the loss of photons resulting from undesirable absorption by contaminants can be alleviated. By these virtues, trace amount of phenol in wastewater can be efficiently mineralized. -
Article
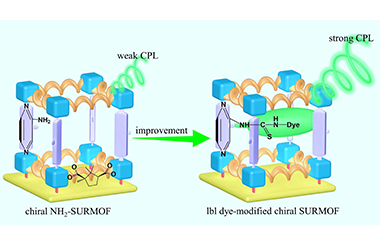
Layer-by-Layer Grafting Dye on Chiral MOF Thin Films for Circularly Polarized Luminescence
Rui Zhai, Yindi Zhu, Limei Chang, Zhigang Gu* and Jian Zhang*
Chin. J. Struct. Chem. 2022, 41, 2209074-2209079 DOI: 10.14102/j.cnki.0254-5861.2022-0023
September 22, 2022
metal-organic frameworks, liquid-phase epitaxy, thin film, circularly polarized luminescence
ABSTRACT
Metal-organic frameworks with chiral feature (chirMOFs) are attracting great attention on circularly polarized luminescence (CPL). However, developing new efficient strategy to achieve or improve CPL properties is an urgent task. Herein, new chiral MOF thin films prepared by liquid-phase epitaxial layer by layer (lbl) growth method (SURchirMOF) are composed of D- or L-camphorate (D/Lcam) and aminopyrazine (Pr-NH2) by using liquid phase epitaxial layer by layer (lbl) method. The resulted Zn2(D/Lcam)2Pr-NH2 SURchirMOF shows strong chirality and luminescence but weak CPL emission at 390 nm. After lbl modifying a dye molecule FluoresceinIsothiocyanate (FITC), the chiroptical Zn2(D/Lcam)2Pr-NH-FITC SURchirMOFs with ~7 times CPL signal improvement and ~3 times glum value amplification are obtained. This work provides a new strategy to develop chiral MOF thin films for CPL improvement using lbl grafting approach. -
Article
FeNC Catalysts with High Catalytic Activity and Stability for Oxygen Reduction Reaction
Yuan-Yuan Feng*, Hua-Shuai Hu, Rui-Jie Liu, Gao Deng, Xiang-Yu Wang and Meng Zhu
Chin. J. Struct. Chem. 2022, 41, 2209080-2209086 DOI: 10.14102/j.cnki.0254-5861.2022-0053
September 22, 2022
FeNC electrocatalyst, pyrolysis, oxygen reduction reaction, methanol tolerance, direct methanol fuel cells (DMFCs)
ABSTRACT
Although Pt-based catalysts have been considered as the most effective electrocatalyst for the cathodic oxygen reduction reaction (ORR) of direct methanol fuel cells (DMFCs), they still suffer from the drawbacks of high cost, poor long-term stability and methanol/ CO poisoning effects. Thus, developing low-cost ORR catalysts with high efficiency, durability and antipoisoning ability is of paramount importance. Herein, a series of non-noble metal FeNC materials are prepared through a facile pyrolysis process and used as the electrocatalysts toward ORR in alkaline electrolyte. Results show that the Fe0.50NC-800-1h catalyst pyrolyzed at 800 ºC for 1 h with the mass ratio of Fe(NO3)3·6H2O to melamine being 0.50 exhibits the highest catalytic performance among the as-prepared FeNC catalysts. The half-wave potential of ORR is ca. 0.81 V, which is only 38 mV lower than that on the noble metal Pt/C catalyst. Besides, it also displays higher stability and methanol tolerance than Pt/C. There is almost no change in the current during the chronoamperometric test when methanol is added in the electrolyte whereas significant decrease is found on Pt/C catalyst. This study of FeNC catalysts provides new insights on understanding the ORR mechanism and suggests a promising strategy to develop low-cost and highly efficient non-noble metal electrocatalysts for ORR.
-
Article
Two Bisligand-Coordinated Luminescent Zn(II)-Coordination Polymers for Sensing of Ions and Pesticides in Aqueous Solutions
Zhao-Di Zhou, Zi-Long Xu, Dan Wang, Lin-Fang Jia, Han-Qing Zhao, Bao-Yi Yu* and Chong-Chen Wang*
Chin. J. Struct. Chem. 2022, 41, 2209087-2209093 DOI: 10.14102/j.cnki.0254-5861.2022-0077
September 22, 2022
coordination polymer, fluorescence sensing, heavy metal ions, pesticides, X-ray crystallography
ABSTRACT
Two coordination polymers (CPs) [Zn(PTA)(DTP)(H2O)2]·(DMF) (CP-1) and [Zn(BTC)(DTP)]·(CH3CN)1.5·(H2O)4 (CP-2) with one- and two-dimensional architectures were synthesized from Zn(II) ion and different organic linkers like terephthalic acid (H2PTA), benzene-1,3,5-tricarboxylic acid (H3BTC), and 3,5-di(1,2,4-triazol-1-yl) pyridine (DTP). The fluorescent sensing experiments showed that the two CPs displayed effective, sensitive, and selective abilities towards Fe3+ and Cr2O72-. For sensing the pesticides, CP-1 outperforms in sensing of metamitron (MMT) and CP-2 is ultrasensitive towards imidacloprid (IMI). The possible mechanisms involved in the quenching of the fluorescence intensity include the inner filter effect (IFE) and the fluore- scence resonance energy transfer (FRET) effect. -
Article
Uncovering the Mechanism for Urea Electrochemical Synthesis by Coupling N2 and CO2 on Mo2C-MXene
Jiahe Peng, Xiao Wang, Zheng Wang, Bin Liu, Peng Zhang, Xin Li* and Neng Li*
Chin. J. Struct. Chem. 2022, 41, 2209094-2209104 DOI: 10.14102/j.cnki.0254-5861.2022-0100
September 22, 2022
urea synthesis, N2 and CO2 coupling, electrocatalysis, MXene, DFT
ABSTRACT
In this work, the catalytic activities of Mo2C-MXene for the co-synthesis of urea from N2 and CO2 are reported by well-defined density functional theory (DFT) method. The calculated results show that the presence of surface functional groups is not conducive to the CO2/N2 (C/N) coupling process in urea synthesis reaction. The exposed Mo2C on the surface can realize urea synthesis at the limit point of 0.69 eV, but the large transition state energy barrier (1.50 eV) indicates that bare Mo2C is not a promising urea catalyst. Loading single atoms can improve the urea synthesis performance of bare Mo2C. The energy barrier of urea synthesis reaction and the transition state energy barrier of C/N coupling reaction have dropped significantly by the atomic loading of Fe and Ti on bare Mo2C. Moreover, Ti doped Mo2C exhibits better catalytic selectivity toward urea production, making it an excellent catalyst for urea synthesis. We hope this work can pave the way for the electrochemical synthesis of urea.
-
Article
Spatially Isolated Noble-Metal-Free Redox Cocatalysts on CdS Nanorods for Increased Photocatalytic Hydrogen Generation
Xiaomeng Zhang, Gege Zhao, Zhongfei Li, Liang Zhu, Yingpeng Cheng, Haiwei Du, Chuhong Zhu, Ya Dang, Daochuan Jiang* and Yupeng Yuan*
Chin. J. Struct. Chem. 2022, 41, 2209105-2209111 DOI: 10.14102/j.cnki.0254-5861.2022-0168
September 22, 2022
spatially isolated cocatalyst, light-induced synthesis, cadmium sulfide, photocatalytic H2 generation
ABSTRACT
Spatially isolated oxidation and reduction cocatalysts on a semiconductor can realize efficient charge separation and thereby lead to increased photocatalytic hydrogen generation. However, the effective preparation of such photocatalysts has proven challenging. Herein, we report the facile synthesis of a novel noble-metal-free CdS/MoS2/CoPi ternary photocatalyst via a visible light-induced synthesis route, in which MoS2 reduction cocatalysts were precisely grown on the two terminals of CdS nanorods, while CoPi oxidation cocatalysts were preferentially anchored onto the sidewalls of CdS nanorods. Such spatially isolated MoS2 and CoPi redox cocatalysts endow CdS nanorods with a rapid charge separation, which enhances their hydrogen generation activity. The CdS/MoS2/CoPi photocatalyst with optimized CoPi amount achieves the highest H2 generation rate of 206 μmol/h, which is 21 and 2 times higher than that achieved by using CdS alone (9.7 μmol/h) and CdS/MoS2 (105 μmol/h), respectively. The present work highlights the effectiveness of the spatial isolation of reduction and oxidation sites for efficient charge separation and thereby provides a promising strategy for the preparation of highly active photocatalysts.