Cover Picture
Unraveling the Dynamic
Structural Evolution of Phthalocyanine Catalysts during CO2 Electroreduction
Jianing Mao, Bingbao Mei*, Ji Li, Shuai Yang, Fanfei Sun,
Siyu Lu, Wei Chen, Fei Song* and Zheng
Jiang*
Phthalocyanine catalysts have well-defined active site structures that allow reaction-based mechanism exploration. In this regard, the actual behaviors of metal ions in the phthalocyanine catalysts have aroused considerable attention. Operando high-energy resolution fluorescence detected X-ray absorption (HERFD-XANES) can be employed in the practical situation of electrocatalysis to realize the interfacial interaction between metal ions and the reactants, offering a unique insight into the active site geometry and structural evolution during CO2 reduction. In this work, the CO2RR to CO dominates over the HER with Faradaic efficiency reaching the maximum value of 89% at 0.85 V versus RHE. The results demonstrate the atomically dispersed, low-valent Ni(I) centres with high intrinsic CO2 reduction activity.
Submit a Manuscript-
Guest Editorial
Preface to Advanced Characterization Technique in Electrocatalysis and Photocatalysis
Zheng Jiang*
Chin. J. Struct. Chem. 2022, 41, 2210001 DOI: 10.14102/j.cnki.0254-5861.2022-0210
October 25, 2022
ABSTRACT
The severe burning of fossil fuels brings about a worsening energy crisis and environmental issues which aroused global concern. Electrocatalysis and photocatalysis are promising approaches to store intermittent renewable solar and wind energy in fuels and chemicals, leading to reduced anthropogenic environmental damage. However, the sluggish kinetics of electrocatalysis and photocatalysis is a huge obstacle to the overall efficiency improvement of energy devices. There is still a knotty problem to rational designing high-performance catalysts, which largely depends on a deep understanding of the interface microenvironment and dynamic structure evolution of catalysts during the reaction. -
News & ViewsProbing Structural Evolution of High-Capacity Layered Oxide Cathode
Yunhui Huang*
Chin. J. Struct. Chem. 2022, 41, 2210002-2210003 DOI: 10.14102/j.cnki.0254-5861.2022-0164
October 25, 2022
ABSTRACT
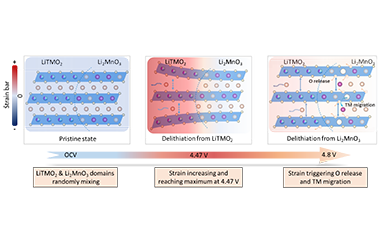
In transitional metal (TM) layered oxide cathode materials, the energy storage relies on Li-ion intercalation/de-intercalation in the Li layers. To achieve higher energy density, lithium- and manganese-rich (LMR) layered oxides are developed by enabling Li-storage in TM layers. Nevertheless, this process involves structural evolution (e.g., oxygen release) of cathode materials that might eventually lead to performance degradation. Typically, LMR materials contain two structures (i.e., LiTMO2 and Li2MnO3) that exhibit different electrochemical behaviors during charging/discharging (Figure 1). Limited by characterization techniques, this process is difficult to observe, leaving the relevant mechanism study a challenge. -
ReviewIn-Situ Synchrotron Radiation Infrared Spectroscopic Identification of Reactive Intermediates over Multiphase Electrocatalytic Interfaces
Wanlin Zhou, Jingjing Jiang, Weiren Cheng, Hui Su* and Qinghua Liu*
Chin. J. Struct. Chem. 2022, 41, 2210004-2210015 DOI: 10.14102/j.cnki.0254-5861.2022-0083
October 25, 2022
synchrotron radiation, infrared spectroscopy, in-situ, reaction dynamics
ABSTRACT
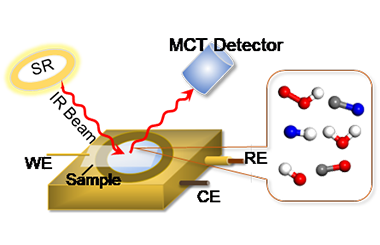
A comprehensive understanding of the microscopic reaction mechanisms at the gas-solid-liquid electrochemical interfaces is urgently required for the development of advanced electrocatalysts applied in burgeoning sustainable energy conversion systems. In-situ synchrotron radiation Fourier transform infrared (SR-FTIR) spectroscopy is one of the most powerful techniques for investigating the evolving dynamics of reactive intermediates during electrocatalytic reactions. In this review, we methodically summarize the recent progress in the research of dynamic mechanisms for valuable electrocatalytic reactions based on in-situ SR-FTIR methodology. Moreover, the merits and drawbacks of SR-FTIR spectroscopy, the design principles of infrared beam setups and in-situ cells, as well as the in-situ measurement criteria are also discussed in detail. Lastly, the potential challenges and opportunities in this field are prudently stated. This review is expected to stimulate a broad interest in the material science and electrochemistry communities for exploring the dynamic mechanisms of prominent catalysis at the atomic/molecular level by using SR-based spectroscopy. -
Review
Soft X-Ray Absorption Spectroscopy of Advanced Two-Dimensional Photo/Electrocatalysts for Water Splitting
Xiaoxin Lv, Guoqing Li, Gaoteng Zhang, Kun Feng, Jiujun Deng* and Jun Zhong*
Chin. J. Struct. Chem. 2022, 41, 2210016-2210028 DOI: 10.14102/j.cnki.0254-5861.2022-0099
October 25, 2022
two-dimensional materials, photocatalyst, electrocatalyst, water splitting, soft XAS
ABSTRACT
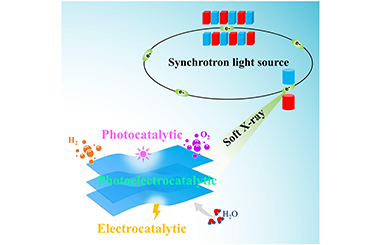
Photo/electrocatalytic water splitting has been considered as one of the most promising approaches for the clean hydrogen production. Among various photo/electrocatalysts, 2D nanomaterials exhibit great potential because of their conspicuous properties. Meanwhile, synchrotron-based soft X-ray absorption spectroscopy (XAS) as a powerful and element-specific technique has been widely used to explore the electronic structure of 2D photo/electrocatalysts to comprehensively understand their working mechanism for the development of high-performance catalysts. In this work, the recent developments of soft XAS techniques applied in 2D photo/ electrocatalysts have been reviewed, mainly focusing on identifying the surface active sites, elucidating the location of heteroatoms, and unraveling the interfacial interaction in the composite. The challenges and outlook in this research field have also been emphasized. The present review provides an in-depth understanding on how soft XAS techniques unravel the correlations between structure and performance in 2D photo/electrocatalysts, which could guide the rational design of highly efficient catalysts for photo/electrocatalytic water splitting. -
ReviewIn-Situ HP-STM and Operando EC-STM Studies of Heterogeneous Catalysis at Interfaces
Lei Xie*, Chaoqin Huang, Zhaofeng Liang, Hongbing Wang, Zheng Jiang and Fei Song*
Chin. J. Struct. Chem. 2022, 41, 2210029-2210044 DOI: 10.14102/j.cnki.0254-5861.2022-0136
October 25, 2022
HP-STM, EC-STM, heterogeneous catalysis, in-situ, operando, interfaces
ABSTRACT
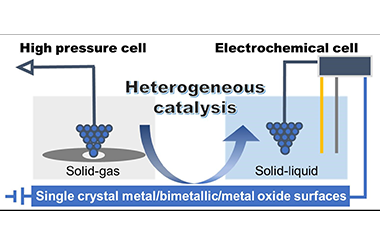
Heterogeneous catalysis taking place at solid interfaces plays a crucial role not only in industrial chemical production, energy conversion but also in fundamental research. The dynamic evolution of surface morphology and composition requires full understanding especially under realistic reaction conditions. To this end, conventional scanning tunneling microscopy (STM) has been integrated with high pressure cell and electrochemical cell, forming high pressure (HP) STM and electrochemical (EC) STM for the in-situ/operando characterization at solid-gas and solid-liquid interfaces with atomic resolution, respectively. In this review, we attempt to give a brief introduction to the development and working principle of these two techniques and subsequently summarize several representative progresses in recent days. The dynamic changes in active sites, surface reconstruction, absorbates alteration and products formation are directly characterized in a combination with other surface sensitive technologies. The correlation between surface structures and catalytic performance as well as the underlying mechanism can thus be unraveled, which provides insights into the rational design and optimization of catalysts. -
Review
Development of In Situ MAS NMR and Its Applications in Material Synthesis and Heterogeneous Catalysis
Xinlong Yao, Zhenchao Zhao* and Guangjin Hou*
Chin. J. Struct. Chem. 2022, 41, 2210045-2210055 DOI: 10.14102/j.cnki.0254-5861.2022-0166
October 25, 2022
in situ NMR, high-temperature high-pressure, material synthesis, heterogeneous catalysis, molecular sieves
ABSTRACT
High-resolution magic angle spinning (MAS) NMR can afford both qualitative and quantitative information of the solid, liquid and gas phase at atomic level, and such information obtained at in situ/operando conditions is of vital importance for understanding the crystallization process of material as well as the reaction mechanism of catalysis. To meet the requirement of experimental conditions for material synthesis and catalytic reactions, in situ MAS NMR techniques have been continuously developed for using at higher temperatures and pressures with high sensitivity. Herein, we will briefly outline the development of this technology and discuss its detailed applications in understanding material synthesis and heterogeneous catalysis.
-
ReviewIn Situ Transmission Electron Microscopy and Three-Dimensional Electron Tomography for Catalyst Studies
Chen Sun, Kuo Liu, Jian Zhang, Qian Liu, Xijun Liu and Lili Han*
Chin. J. Struct. Chem. 2022, 41, 2210056-2210076 DOI: 10.14102/j.cnki.0254-5861.2022-0187
October 25, 2022
in situ TEM, catalyst, electron tomography, 3D reconstruction, artificial intelligence, machine learning
ABSTRACT
An in-depth understanding of the catalytic reaction mechanism is the key to designing efficient and stable catalysts. In situ transmission electron microscope (TEM) is the most powerful tool to visualize and analyze the microstructures of catalysts during catalysis. In situ TEM combined with three-dimensional (3D) electron tomography (ET) reconstruction technique enables interrogations of catalysts' structural dynamics and chemical changes in high temporal and spatial dimensions. In this review, we discuss and summarize the recent advances in in situ TEM together with 3D ET for catalyst studies. Topics include the latest research progress of in situ TEM imaging as well as 3D visualization and quantitative analysis of catalysts. We also pay particular attention to artificial intelligence (AI)-enhanced smart 3D ET. These include deep learning (DL)-based data compression and storage for the analysis of large TEM data, recovery of wedge-shaped information lost in 3D ET reconstructions, and DL models for reducing residual artifacts in 3D reconstructed images. Finally, the challenges and development prospects of current in situ TEM and 3D ET research are discussed. -
CommunicationAn In-Situ Variable-Temperature Surface-Enhanced Raman Spectroscopic Study of the Plasmon-Mediated Selective Oxidation of p-Aminothiophenol
Wumei Cao, Yang Lu and Yi-Fan Huang*
Chin. J. Struct. Chem. 2022, 41, 2210077-2210081 DOI: 10.14102/j.cnki.0254-5861.2022-0134
October 25, 2022
surface-enhanced Raman spectroscopy, p-aminothiophenol, temperature, plasmon-mediated chemical reaction
ABSTRACT
The roles of temperature change in surface-enhanced Raman scattering (SERS) hotspots are important for understanding the plasmon-mediated selective oxidation of p-aminothiophenol in a SERS measurement. Here, we demonstrate that the temperature change in hotspots seriously influences the conversion of p-aminothiophenol on Au by employing variable-temperature SERS measurements. The conversion steadily and irreversibly increased when the temperature increased from 100 to 360 K. But the conversion decreased above 360 K, because this conversion was exothermic. This temperature-dependence conversion suggests that the temperature change in hotspots originated from the photothermal effect should be coupled to the hot-electron effect in promoting the selective oxidation of p-aminothiophenol. -
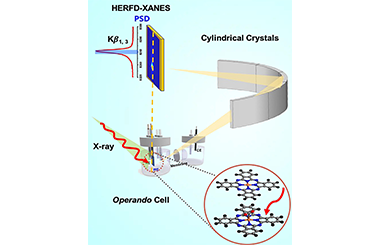
ArticleUnraveling the Dynamic Structural Evolution of Phthalocyanine Catalysts during CO2 Electroreduction
Jianing Mao, Bingbao Mei*, Ji Li, Shuai Yang, Fanfei Sun, Siyu Lu, Wei Chen, Fei Song* and Zheng Jiang*
Chin. J. Struct. Chem. 2022, 41, 2210082-2210088 DOI: 10.14102/j.cnki.0254-5861.2022-0133
October 25, 2022
CO2 electrochemical reduction, HERFD-XAS, model catalysts, difference spectra, structural evolution
ABSTRACT
Understanding the atomic and electronic changes of active sites promotes the whole new sight into electrochemical carbon dioxide reduction reaction (CO2RR), which provides a feasible strategy to achieve carbon neutrality. Here we employ operando high-energy resolution fluorescence-detected X-ray absorption spectroscopy (HERFD-XAS) to track the structural evolution of Ni(II) phthalocyanine (NiPc), considered as the model catalysts with uniform Ni-N4-C8 moiety, during the CO2RR. The HERFD-XAS method is in favor of elucidating the interaction of the reactant/catalyst interface from the atomic electronic structure dimension, facilitating the establishment of the catalytic mechanism and the dynamic structure changes. Based on operando measurement, surface sensitive difference spectra (∆µ) and spectroscopy simulation, the interfacial interactions between the active sites of NiPc and reactants are monitored and the Ni species gradually reduced by increasing the applied potential is discovered. HERFD-XAS method offers an advanced and powerful tool for elucidating the complex catalytic mechanism in further various systems.