Cover Picture
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
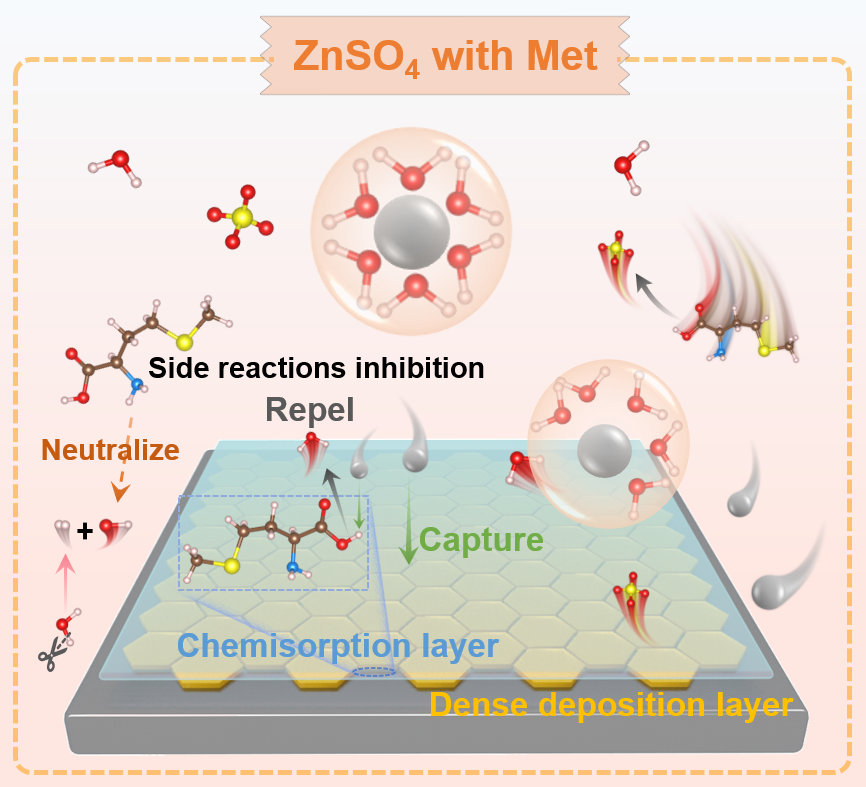
Microenvironment regulation of anode-electrolyte interface enables highly stable Zn anodes
Lin Peng, Xincheng Liang, Zelong Sun, Xingfa Chen, Dexin Meng, Renshu Huang, Qian Liu, Huan Wen, Shibin Yin*
Chin. J. Struct. Chem., 2025, 44(4), 100542. DOI: 10.1016/j.cjsc.2025.100542
April 1, 2025
Aqueous zinc ion batteries; Zn anodes; Electrolyte additives; Anode-electrolyte interface; Capture effect; pH buffer
ABSTRACT
H2O-induced side reactions and dendrite growth occurring at the Zn anode-electrolyte interface (AEI) limit the electrochemical performances of aqueous zinc ion batteries. Herein, methionine (Met) is introduced as an electrolyte additive to solve the above issues by three aspects: Firstly, Met is anchored on Zn anode by amino/methylthio groups to form a H2O-poor AEI, thus increasing the overpotential of hydrogen evolution reaction (HER); secondly, Met serves as a pH buffer to neutralize the HER generated OH−, thereby preventing the formation of by-products (e.g. Zn4SO4(OH)6⋅xH2O); thirdly, Zn2+ could be captured by carboxyl group of the anchored Met through electrostatic interaction, which promotes the dense and flat Zn deposition. Consequently, the Zn||Zn symmetric cell obtains a long cycle life of 3200 h at 1.0 mA cm−2, 1.0 mAh cm−2, and 1400 h at 5.0 mA cm−2, 5.0 mAh cm−2. Moreover, Zn||VO2 full cell exhibits a capacity retention of 91.0% after operating for 7000 cycles at 5.0 A g−1. This study offers a novel strategy for modulating the interface microenvironment of AEI via integrating the molecular adsorption, pH buffer, and Zn2+ capture strategies to design advanced industrial-oriented batteries.