Cover Picture
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
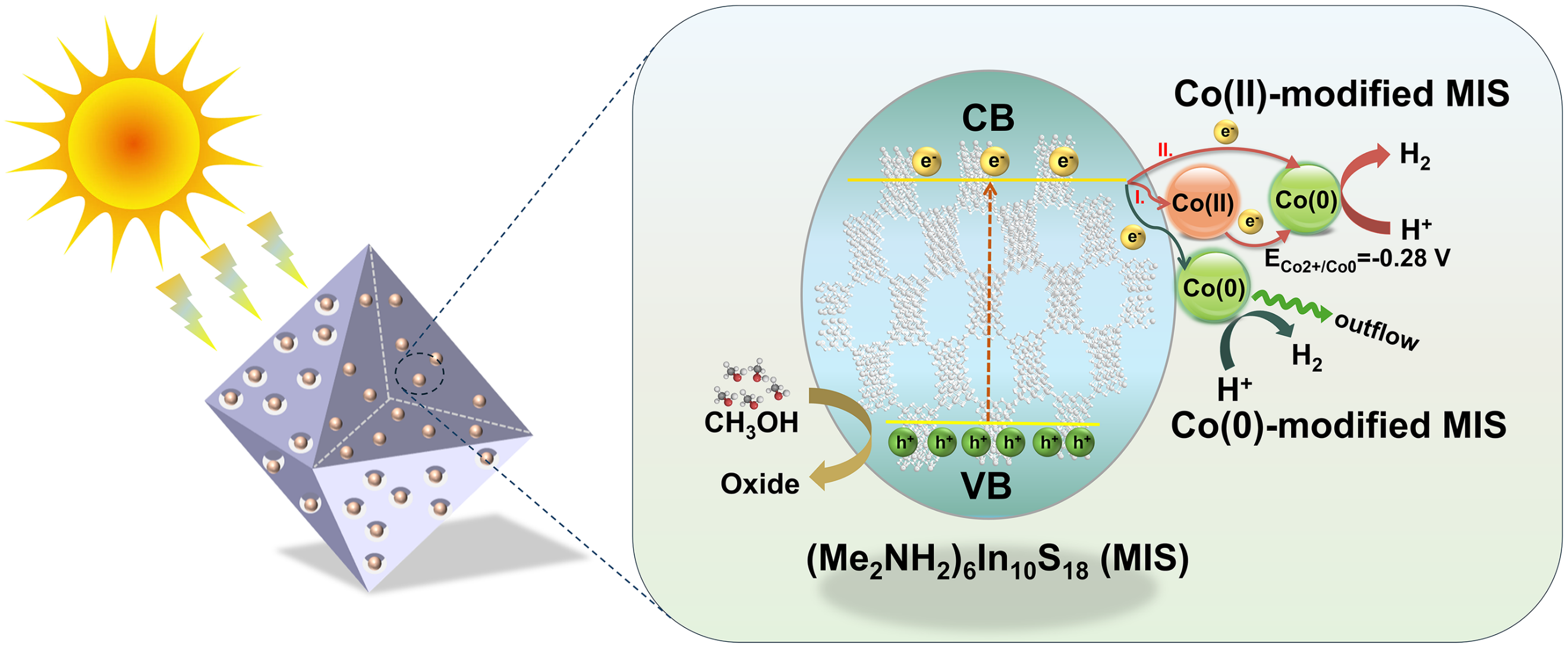
Novel open-framework chalcogenide photocatalysts: Cobalt cocatalyst valence state modulating critical charge transfer pathways towards high-efficiency hydrogen evolution
Haiyan Yin, Abdusalam Ablez, Zhuangzhuang Wang, Weian Li, Yanqi Wang, Qianqian Hu*, Xiaoying Huang
Chin. J. Struct. Chem., 2025, 44(4), 100560. DOI: 10.1016/j.cjsc.2025.100560
April 1, 2025
Open-framework Metal sulfide; Ion exchange; Cobalt cocatalyst; Valence state; Charge transfer; Photocatalytic hydrogen evolution
ABSTRACT
Negatively charged open-framework metal sulfides (NOSs), taking advantages of the characteristics of excellent visible light absorption, easily exchanged cations, and abundant active sites, hold significant promise as highly efficient photocatalysts for hydrogen evolution. However, their applications in photocatalytic hydrogen evolution (PHE) are infrequently documented and the corresponding photocatalytic mechanism has not yet been explored. Herein, we excavated a novel NOS photocatalyst of (Me2NH2)6In10S18 (MIS) with a three-dimensional (3D) structure, and successfully incorporated divalent Co(II) and metal Co(0) into its cavities via the convenient cation exchange-assisted approach to regulate the critical steps of photocatalytic reactions. As the introduced Co(0) allows for more efficient light utilization and adroitly surficial hydrogen desorption, and meanwhile acts as the ‘electron pump’ for rapid charge transfer, Co(0)-modified MIS delivers a surprising PHE activity in the initial stage of photocatalysis. With the prolonging of illumination, metal Co(0) gradually escapes from MIS framework, resulting in the decline of PHE performance. By stark contrast, the incorporated Co(II) can establish a strong interaction with MIS framework, and simultaneously capture photogenerated electrons from MIS to produce Co(0), which constructs a stable photocatalytic system as well as provides additional channels for spatially separating photogenerated carriers. Thus, Co(II)-modified MIS exhibits a robust and highly stable PHE activity of ∼4944 μmol/g/h during the long-term photocatalytic reactions, surpassing most of the previously reported In–S framework photocatalysts. This work represents a breakthrough in the study of PHE performance and mechanism of NOS-based photocatalysts, and sheds light on the design of guest confined NOS-based photocatalysts towards high-efficiency solar-to-chemical energy conversion.