Development of a theranostic tri-nuclear gadolinium(III) complex based on apoferritin for multitarget therapy of orthotopic glioma
Xueyu Man, Guochao Li, Minghui Zhu, Shanhe Li, Gang Xu, Zhenlei Zhang*, Hong Liang, Feng Yang* Submit a Manuscript
You-Song Ding*, Qing-Song Yang, Zhiping Zheng*
Chin. J. Struct. Chem., 2025, 44(11), 100696. DOI: 10.1016/j.cjsc.2025.100696
November 1, 2025
ABSTRACT
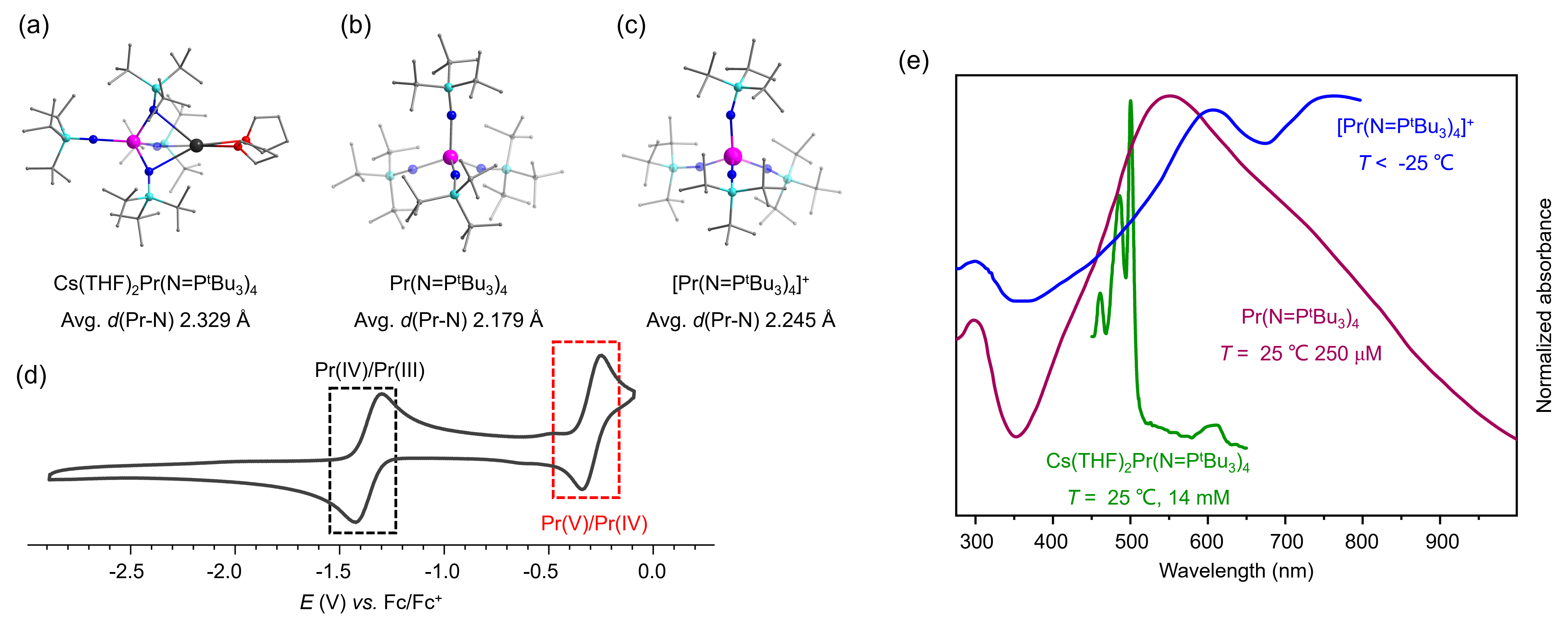
To summarize, the first isolable pentavalent lanthanide complexes were obtained by chemical oxidation of a tetravalent complex at low temperature. Their crystal structures have been determined by single-crystal X-ray diffraction studies, while the formal +5 oxidation state of the metal ion is established by the combined use of electrochemical and spectroscopic analyses. The experimental results are supported by computational studies of the electronic structures of the complexes. This work expands the landscape of the redox chemistry of the lanthanides and provides a critical framework for understanding high-valence metal bonding across the periodic table. Future efforts may be directed toward 1) making Pr(V) complexes with other types of ligands to tune the electronic structures; 2) assessing their catalytic potentials; 3) exploring analogous high-valence-state chemistry for other early lanthanide elements (e.g. Nd, Sm); and 4) developing advanced theoretical models for accurate description of multiconfigurational f-element systems.