Structural optimization of organometallic cages for enhanced photothermal solar water evaporation
Xiao-Qian Wan, Ya-Ning Xu, Jian-Xin Yang, Dan Tian*, Li-Long Dang*, Feng Bai, Lu-Fang Ma
Chin. J. Struct. Chem., 2025, 44(10), 100705. DOI: 10.1016/j.cjsc.2025.100705
October 15, 2025
Organometallic cage; Half-sandwich unit; Coordination-driven self-assembly; Photothermal conversion
ABSTRACT
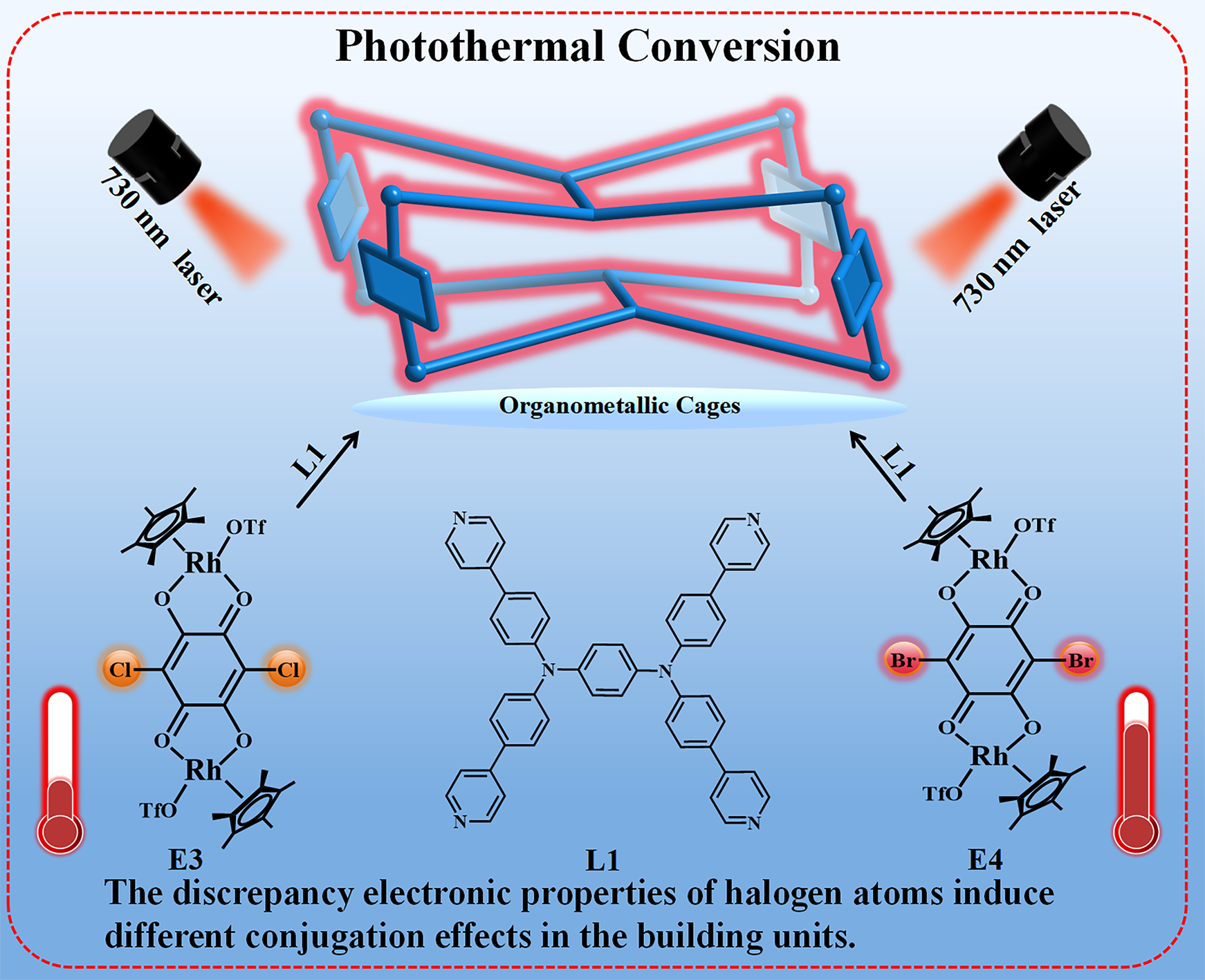
The structural synthesis and property exploration of organometallic cages has always attracted widespread attention from chemists. Nevertheless, the achievement on photothermal property enhancement and their application in solar-driven water evaporation via structural modulation remains scarce. Here, four organometallic cages 1, 2, 3 and 4 with different functional sites are synthesized via reasonably selecting different building units E1, E2, E3 and E4 based on a tetradentate pyridyl ligand L1. These complexes are characterized by single-crystal X-ray diffraction analysis, nuclear magnetic resonance (NMR) spectroscopy and ESI-TOF-MS analysis. Notably, they exhibit different near-infrared (NIR) photothermal conversion properties due to variations in their size, conjugated area, electron-withdrawing characteristic of halogen atoms in building units. Compound 4 shows the optimal photothermal performance among the series, with notably enhanced near-infrared absorption and the highest photothermal conversion efficiency. The radical effect of the building unit plays an important role in photothermal conversion ability as evidenced by the significant EPR signal changes. Therefore, compound 4 is used to construct new membrane 1', achieving a solar power-induced water steam generation rate of 1.92 kg·m-2·h-1, demonstrating its suitability for the collection of fresh water through desalination and wastewater treatment.. This research provides a new strategy for synthesizing and optimizing photothermal conversion property of half sandwich organometallic cages.