Cover Picture
Nuclear magnetic resonance-guided Monte Carlo/molecular dynamics structure inference for amorphous solids
Kangren Kong, Zaiqiang Ma, Yu Yin, Zhaoming Liu, Ruikang Tang* Submit a Manuscript
Nuclear magnetic resonance-guided Monte Carlo/molecular dynamics structure inference for amorphous solids
Kangren Kong, Zaiqiang Ma, Yu Yin, Zhaoming Liu, Ruikang Tang* Submit a Manuscript
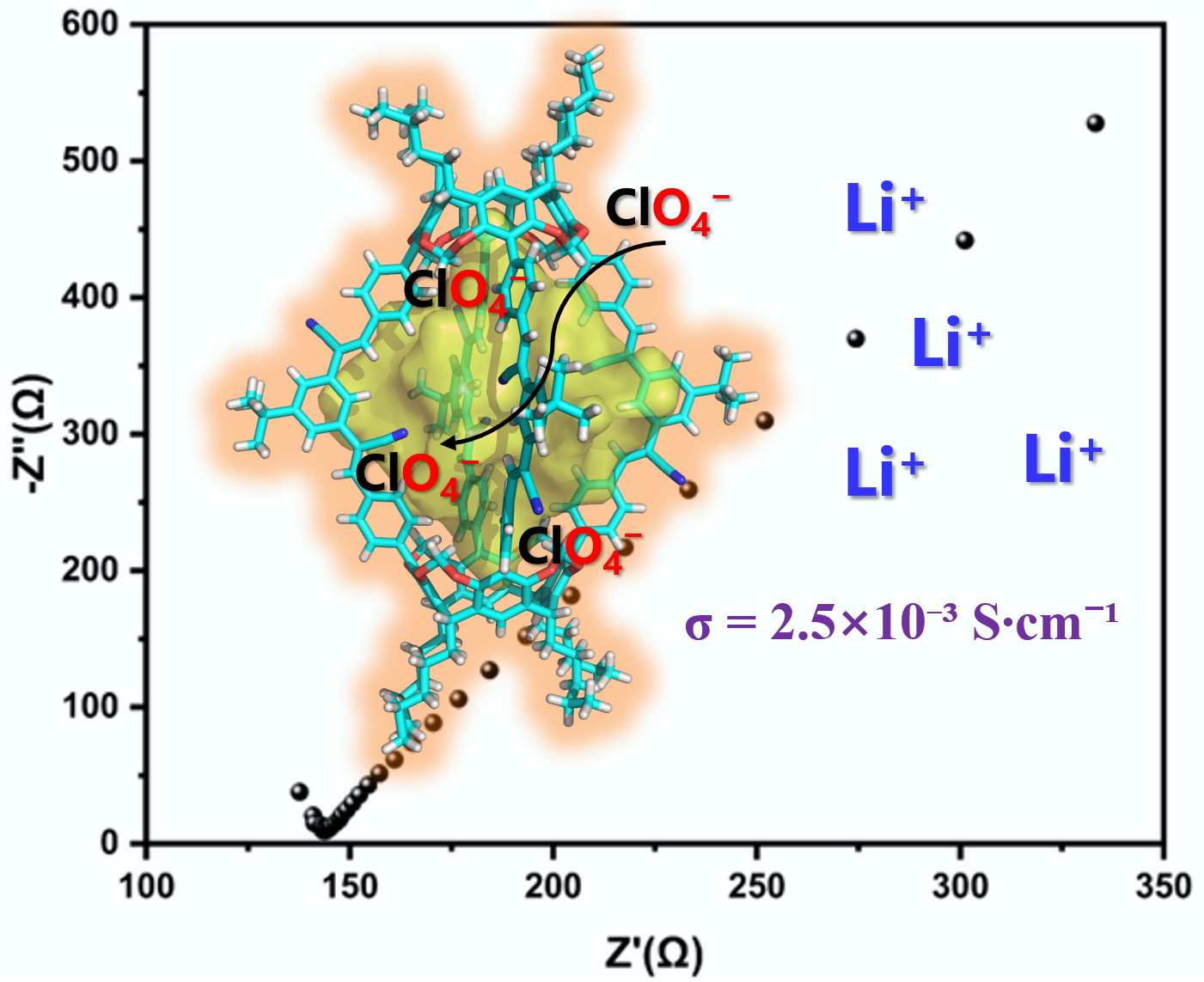
Ultrastable sp2 carbon-conjugated porous organic cage for selective per-chlorate capture and fast lithium‑ion transport
Fenglei Qiu†, Xinting Zhang†, Fengquan Liu, Linfeng Liang*, Wenjing Wang, Kongzhao Su*, Daqiang Yuan*
Chin. J. Struct. Chem., 2026, 45(3), 100843. DOI: 10.1016/j.cjsc.2025.100843
March 1, 2026
Porous organic cage; Calix[4]resorcinarene; Perchlorate capture; Host-guest interaction; Lithium-ion transport
ABSTRACT
Organic cage compounds, which are among the most important classes of supramolecular hosts, have been found to be capable of capturing various guests through host-guest interactions due to their inherent cavities. To date, the exploration of potential applications based on such host-guest chemistry has been a subject of intensive research. Herein, we report a highly stable sp2 carbon-conjugated porous organic cage (POC), abbreviated as sp2c-POC3, formed via the Knoevenagel reaction between tetraformyl-functionalized calix[4]resorcinarene and V-shaped diacetonitrile subunits. X-ray crystallographic analysis reveals that sp2c-POC3 is a [2+4] long lantern-shaped cage. It contains four rhombic windows with an average edge length of approximately 2.1 nm and a large cavity with a volume of approximately 782 Å3. Notably, this cage can selectively capture perchlorate (ClO4-) anions. Taking advantage of such anion trapping ability and the porous nature, a quasi-solid-state electrolyte (QSSE) based on sp2c-POC3 and incorporating LiClO4 has been rationally designed. This sp2c-POC3-based QSSE exhibits a high ionic conductivity of 2.5×10-3 S cm-1 at room temperature.