Mechanism Study of Aliskiren and Its Analogues by Molecular Dynamic Simulation
YAN Wen-Li, LIANG Zhen, YU Xing-Lian and ZHANG Rong*
Chin. J. Struct. Chem. 2022, 41, 2203178-2203185 DOI: 10.14102/j.cnki.0254-5861.2011-3308
March 15, 2022
renin inhibitors, mechanism, molecular docking, molecular dynamics (MD) simulations
ABSTRACT
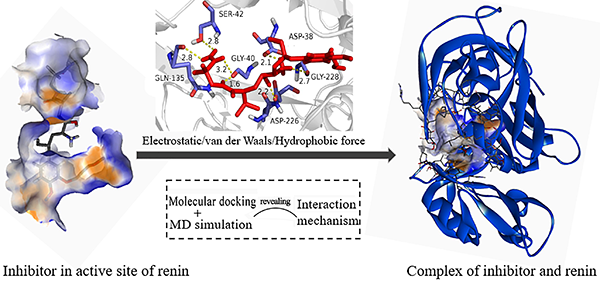
The further interaction mechanism towards renin
inhibitors was revealed by comparison of renin with different active inhibitors
in aqueous solution. Molecular docking and molecular dynamics (MD) simulations
were combined for the research. The results reflected that electrostatic and
hydrophobic effects were the major interactions for renin inhibitors forming
complexes with renin, and some residues were the key to the formation of
complex, especially Asp38/Asp226. The factor of different activities performed
in renin inhibitors was illustrated as well. For the higher active renin
inhibitor, it possessed stronger affinity with renin, and its detected conformation
was more extended to fit for the key binding site. This promoted the capacity
to form special interactions with the key residues. While conformation of the
lower active renin inhibitor performed folded in the active site of renin, the
interactions to the important pocket S3sp was restricted, resulting in
undesirable bioactivity.