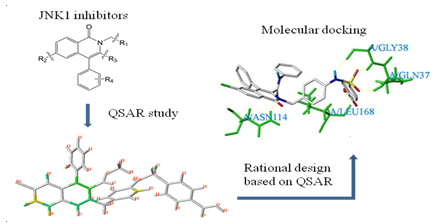
QSAR Study and Molecular Design of Isoquinolone Derivative JNK1 Inhibitors
TONG Jian-Bo*, XIAO Xue-Chun, LUO Ding, XU Hai-Yin and WANG Jie
Chin. J. Struct. Chem. 2021, 40, 1586-1594 DOI: 10.14102/j.cnki.0254-5861.2011-3227
December 15, 2021
Topomer CoMFA, HQSAR, molecular docking, isoquinolone derivatives, molecular design
ABSTRACT
JNK1
is a drug target for the treatment of type 2 diabetes, and it plays a key
mediator role in metabolic disorders. In this paper, holographic quantitative structure-activity
relationship (HQSAR) technology and Topomer comparative molecular field analysis (Topomer CoMFA) technology are used to analyze the
quantitative structure-activity relationship (QSAR) of 39 isoquinolone
derivatives. The cross validation correlation coefficient (q2) is 0.696 (Topomer CoMFA) and 0.826 (HQSAR), and the non-cross
validation correlation coefficient (r2)
is 0.935 (Topomer CoMFA) and 0.987 (HQSAR). The results showed that the models have
good stability and predictive ability. The Topomer search module was applied to
define high contribution fragments in the ZINC database, designing 20 new
isoquinolone compounds with theoretically high inhibitory activity. The molecular
docking was carried out to explore the interaction between the ligand and
target JNK1 protein. This study can provide a theoretical basis for the design
of new JNK1 inhibitors.