Synthesis and Absolute Configuration of ((2R,3R,6S)-3-Hydroxy-6-(naphthalen-2-ylthio)- 3,6-dihydro-2H-pyran-2-yl)methyl Pivalate
JIAO Yang, ZHANG Jia-Ding, WANG Shu-Zhang, YAO Hui, LIU Ming-Guo* and HUANG Nian-Yu*
Chin. J. Struct. Chem. 2021, 40, 1238-1245 DOI: 10.14102/j.cnki.0254-5861.2011-3124
September 15, 2021
X-ray diffraction, crystal structure, absolute configuration,β-thiogalactoside, cytotoxity
ABSTRACT
A stereo-selective palladium-catalyzed one-pot Tsuji-Trost
reaction was used to prepare four β-thiogalactosides
from unsaturated D-galactal and thiol. Their structures were characterized by nuclear
magnetic resonance spectra and high-resolution electrospray ionization mass
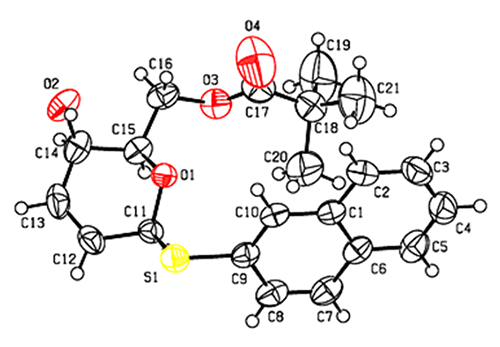
spectra. The absolute configuration was confirmed with a Flack parameter of 0.019(15)
by X-ray crystallography using a Cu radiation source. Compound 6a (C21H24O4S): orthorhombic system, space group P212121, a = 9.0919(4), b = 9.6313(4), c = 22.5936(11) Å, V = 1978.45(15) Å3, Z = 4, F(000) = 792, Dc = 1.250 g/cm3, μ = 1.636 mm−1, R = 0.0478 and wR = 0.1384 for 3621 independent reflections (Rint = 0.0390)
and 3326 observed ones (I > 2σ(I)). 3-(4,5)-Dimethylthiazol-2-yl-2,5-
diphenyltetrazolium bromide (MTT) cell viability assays indicated that these thiogalactosides showed anti-proliferative
activities against human gastric cancer HGC-27 cells with IC50 values of 69~88 μM.