Microwave-assistant Syntheses, Crystal Structures and Safener Activities of Two Substituted Phenyl Isoxazole Derivatives
GAO Ying-Chao, SHAO Xin-Xin and FU Ying*
Chin. J. Struct. Chem. 2021, 40, 1231-1237 DOI: 10.14102/j.cnki.0254-5861.2011-3142
September 15, 2021
isoxazole benzoxazine formamide, microwave-assistant synthesis, single-crystal structure, safener activity
ABSTRACT
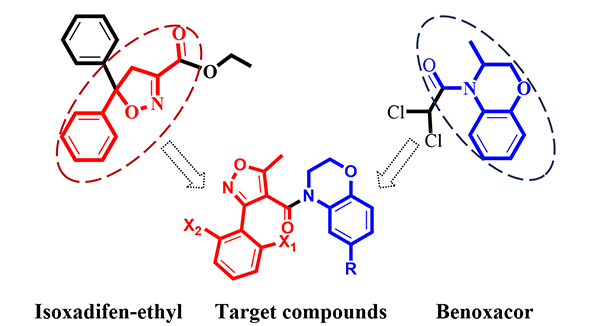
Two novel substituted phenyl isoxazole benzoxazine formamide derivatives were designed and synthesized with substituted o-aminophenol, 1,2-dibromoethane and different phenyl substituted
isoxazole formyl chloride as the raw materials via microwave assistant synthesis. The target compounds were characterized by IR, 1H NMR, 13C
NMR and HRMS. Both single-crystal structures were further determined by X-ray diffraction. 3-(2΄-Chloro-6΄-fluoro-phenyl)-4-(2΄,3΄-dihydro-1΄,4΄-benzoxazine)-5-methyl-isoxazole formamide (4a) crystallizes in
orthorhombic system, P21 space group with a = 8.9414(18), b = 10.834(2), c = 17.706(4) Å, V =
1715.1(6) Å3, Dc = 1.444 Mg/m3, Z = 4, F(000) = 768, m(MoKa) = 0.255 mm-1, R = 0.0406 and wR =
0.1171. 3-Phenyl-4-(6- methyl-2΄,3΄-dihydro-1΄,4΄-benzoxazine)-5-methyl-isoxazole
formamide (4b) is of triclinic system, space group P
with a = 7.7659(16), b = 8.3626(17), c =
13.484(3) Å, a = 76.04(3)°, b = 100.63(3)°, g = 82.01(3)°, V = 841.6(3) Å3, Dc = 1.319 Mg/m3, Z = 2, F(000) = 352, m(MoKa) = 0.090 mm-1, R = 0.0672 and wR =
0.2671. Both crystals are packed through C–H···O hydrogen bonding interaction. There
is C–H···F hydrogen bond between 4a molecules, and C–H···N between 4b molecules. Bioassay results showed
that compounds 4a and 4b exhibited detoxification on maize
and restored maize growth index.