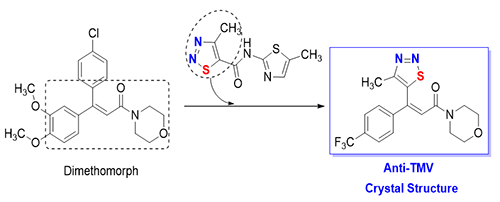
Synthesis, Crystal Structure and Anti-TMV Activity of (Z)-4-[3-(4-Methyl-1,2,3-thiadiazol-5-yl)-3-(4-trifluoromethylphenyl)acryloyl]morpholine
SUN Su-Su, LI Qing, GAO Wei, LI Xiao-Tian, CHEN Lai* and ZHANG Jin-Lin*
Chin. J. Struct. Chem. 2021, 40, 109-113 DOI: 10.14102/j.cnki.0254-5861.2011-2753
January 15, 2021
1,2,3-thiadiazole, cinnamic amide, synthesis, crystal structure, anti-TMV activity
ABSTRACT
The
target compound (Z)-4-[3-(4-methyl-1,2,3-thiadiazol-5-yl)-3-(4-trifluoromethylphenyl)acr-
yloyl]morpholine was
synthesized by the nucleophilic substitution, Horner-Emmons reaction, ester
hydrolysis, and condensation. Its structure was characterized by NMR, H RMS and single-crystal X-ray diffraction. The crystal of the target
compound belongs to monoclinic system, space group P21 with a = 11.5058(15), b = 6.6626(10), c = 23.184(3)
Å, V = 1777.3(4) Å3, Z = 8, Dc = 1.496 Mg/m3, F(000) = 792 and μ = 0.229
mm–1.
X-ray analysis indicated
C–H×××O intermolecular H-bonds
in this crystal structure. The target compound exhibited 53% curative activity
against TMV.