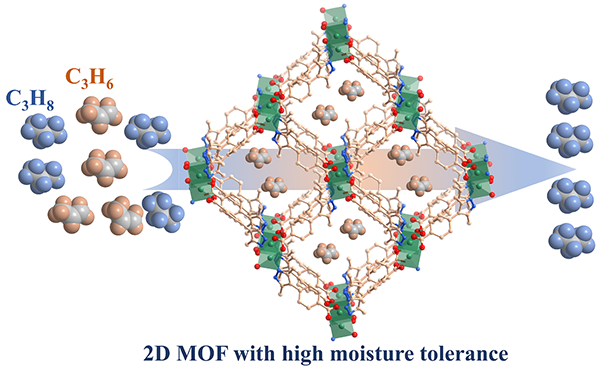
Robust microporous metal-organic framework with high moisture tolerance for efficient separation of propylene from propane
Shan-Qing Yang, Lei Zhou, Bo Xing, Ying-Hui Zhang, Tong-Liang Hu*
Chin. J. Struct. Chem., 2023, 42: 100004. DOI: 10.1016/j.cjsc.2022.100004
February 15, 2023
Metal-organic framework, Adsorptive separation, Propylene/propane separation, Energy efficient
ABSTRACT
Adsorptive separation of propylene (C3H6) from propylene/propane (C3H8) is desired to replace energy-intensive cryogenic distillation for its energy efficiency, economical viability, and environment friendship. In this work, we report a cupper-based robust microporous metal-organic framework, Cu-Hmpba, constructed by the hard soft acid-base principle using the bifunctional pyridylcarboxylate ligand featuring methyl group, for high-efficient separation of the propylene/propane mixture. Under the synergistic effect of the protective group and hard soft acid base principle, Cu-Hmpba possesses good solvents stability, especially water stability. Cu-Hmpba exhibits the adsorption capacity of C3H6 with 2.10 mmol g−1 and good equimolar C3H6/C3H8 selectivity with 2.24 at ambient conditions. The gas adsorption experiments, IAST selectivity calculations and theoretical calculations comprehensively support the selective adsorption toward C3H6 among C3H6/C3H8 mixture. Furthermore, it could maintain stable under moisture environment, suggesting its potential for the industrial separation of C3H6/C3H8 mixture. The results of experiments and simulations demonstrate that the Cu-Hmpba would be a candidate adsorbent for the separation and purification of light hydrocarbons, and this work provides the insight of synthesizing stable MOF materials for separating high-value chemicals.