Cover Picture
Tandem electrocatalytic nitrate reduction reaction
Chuanfei Cang, Haoquan Zheng* Submit a Manuscript
Tandem electrocatalytic nitrate reduction reaction
Chuanfei Cang, Haoquan Zheng* Submit a Manuscript
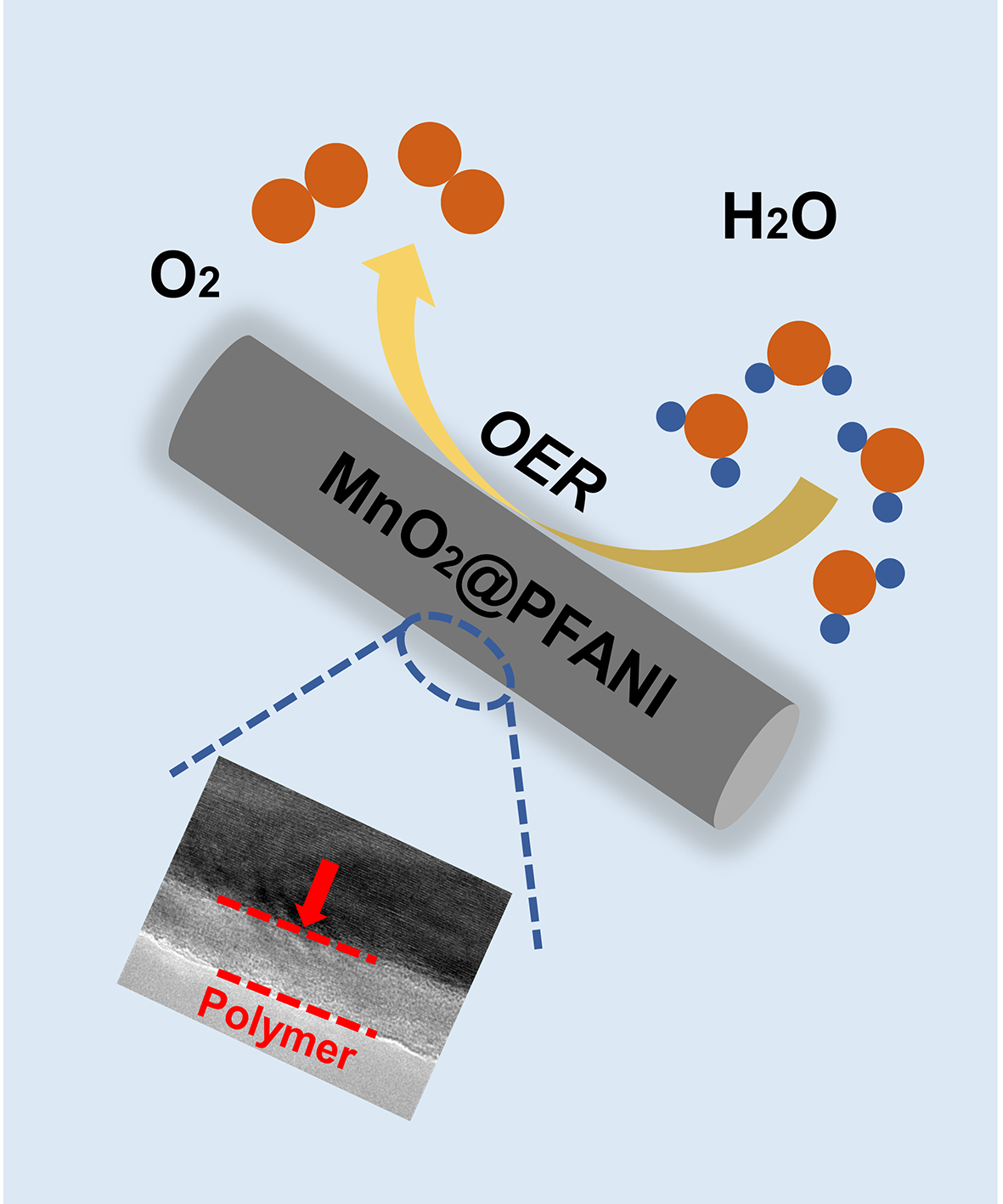
Hydrophilic MnO2 nanowires coating with o-fluoroaniline for electrocatalytic water oxidation
Lingshuang Qin, Wei Zhang*, Rui Cao*
Chin. J. Struct. Chem., 2023, 42: 100105. DOI: 10.1016/j.cjsc.2023.100105
August 15, 2023
Electrocatalysis; Oxygen evolution reaction; MnO2; Hydrophilicity; Conductivity
ABSTRACT
In the active Mn4CaO5 cluster of the natural water oxidation system, one of the dangling Mn atoms is surrounded by water molecules through coordination and hydrogen bonding. It means that the enrichment of water molecules around the Mn catalysts has a positive effect on the water oxidation catalysis. Inspired by nature photosynthesis, the hydrophilic surfaces are believed to be able to capture water molecules to assist water oxidation. Herein, we report MnO2@PFANI nanowires with improved mass transfer efficiency and conductivity for electrocatalytic water oxidation. The as-prepared nanowires are synthesized by coating hydrophilic polymers on MnO2 nanowires. The hydrophilic surface captures water molecule and promotes mass transfer processes, and the conductivity of the polymer can also increase charge transfer processes. To reach a current density of 10 mA cm−2, a low overpotential of 440 mV is required in a 1.0 M KOH solution for the MnO2@PFANI nanowires. The hydrophilicity and conductivity are systematically investigated by both physical and electrochemical measurements. This study provides a surface engineering idea for the fabrication of efficient electrocatalysts.