Ruiqi Gao, Huan He, Junxian Bai, Lei
Hao,
Rongchen Shen*, Peng Zhang, Youji Li and Xin Li*
The blue cover, together with elements such as blisters, lightning and the sun, highlights the good prospect of this new type of heterojunction as a photocatalytic material. The "S" shaped dragon depicted by the element of water rises from the bottom, implying that the dragon gets water and magically turns water into green hydrogen over organic/inorganic S-type heterojunction photocatalyst under sunlight irradiation.
Enhanced Photocatalytic H2-production Activity of CdS Nanoflower using Single Atom Pt and Graphene Quantum Dot as Dual Cocatalysts
Yi Yang, Jinsong Wu, Bei Cheng*, Liuyang Zhang*, Ahmed Abdullah Al-Ghamdi, Swelm Wageh and Youji Li
Chin. J. Struct. Chem. 2022, 41, 2206006-2206014 DOI: 10.14102/j.cnki.0254-5861.2022-0124
June 20, 2022
hierarchical nanostructure, single-atom catalysts, graphene quantum dots, CdS nanosheets, hydrogen production
ABSTRACT
Single-atom catalysts have high catalytic activity due to their unique
quantum size effects and optimal atom utilization. Herein, visible-light-responsive photocatalysts were
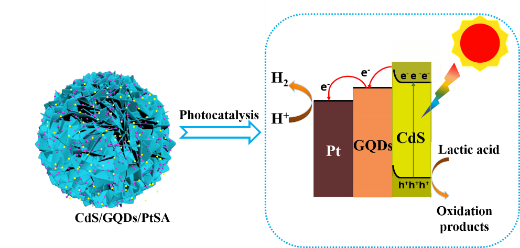
designed by coupling CdS with graphene quantum dots (GQDs) and platinum single atoms
(PtSAs). GQDs and PtSAs were successively loaded on ultrathin
CdS nanosheets through freeze-drying and in-situ photocatalytic reduction. The
synergistic effect between PtSAs and GQDs results in superior photocatalytic
activity with a hydrogen production rate of 13488 μmol h-1 g-1 as well as the maximum apparent quantum efficiency (AQE) of 35.5% in lactic
acid aqueous solution, which is 62 times higher than that of pristine CdS (213
μmol g-1 h-1). The energy conversion efficiency is ca.
13.05%. As a photosensitizer and an electron reservoir, GQDs can not only
extend the light response of CdS to the visible-light region (400-800 nm), but
also promotes the separation of photoinduced electron-hole pairs. Meanwhile,
PtSAs, with unique electronic and geometric features, can provide more
efficient proton reduction sites. This finding provides an effective strategy
to remarkably improve photocatalytic H2 production performance.