-
Guest Editorial
Preface to Solar Photocatalysis
Jiaguo Yu*, Xin Li*, Zhiliang Jin*, Hua Tang* and Enzhou Liu*
Chin. J. Struct. Chem. 2022, 41, 2206001-2206002
June 20, 2022
Solar Photocatalysis
ABSTRACT
Solar energy is the most important clean and renewable energy in the world. However, the unpredictability, seasonal variation day and night, uneven distribution and low energy density limit its practical application. Photocatalysis technology has a very broad application prospect in solving energy and environmental problems. Photocatalysis can use sunlight to decompose water to produce hydrogen, and convert light energy into storable chemical energy-hydrogen energy. Hydrogen energy has high energy density and is considered as an ideal energy carrier. Photocatalysis can also use sunlight to convert carbon dioxide into methane and methanol fuel to achieve the purpose of energy conservation and emission reduction. However, because of low photocatalytic efficiency, the energy and environmental applications of various photo- catalytic materials and technologies are still very limited. Thus, various improvement and investigations are highly required from the viewpoint of practical utilization. -
HighlightMolecular-Level Engineering of S-scheme Heterojunction: the Site-Specific Role for Directional Charge Transfer
Jianjun Zhang, Linxi Wang*, Mitra Mousavi, Jahan B. Ghasemi and Jiaguo Yu
Chin. J. Struct. Chem. 2022, 41, 2206003-2206005 DOI: 10.14102/j.cnki.0254-5861.2022-0150
June 20, 2022
S-scheme heterojunction, functionalized Co-doped regulation, directional electron-driving effect, CO2 photoreduction
ABSTRACT
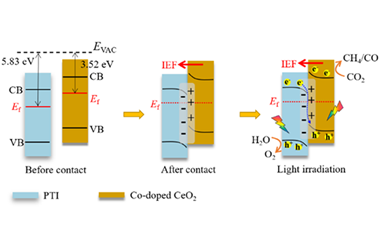
The promising S-scheme heterojunction photocatalysts are considered as a novel frontier due to their superiority in various solar-driven energy-related applications. Recently, a novel atom-specific tailoring strategy has been introduced on the construction of S-scheme heterojunction for promoting the electronic transferability. The S-scheme heterojunction is regulated by integrating high-crystalline carbon nitride with Co-doped CeO2. Specifically, this atom-specific regulation of S-scheme heterojunction boosts directional electron-driving effect towards functionalized Co sites, benefit-ing for effective photogenerated charge carrier transferability. Moreover, a series of tracking characterizations show that Co-embedded modification promotes CO2 photoreduction into hydrogenation steps, resulting in high performance towards CO2-to-CH4 photoreduction, which provides new opportunities for the development of multifunctional cooperation in heterogeneous photocatalysis.
-
Article
Enhanced Photocatalytic H2-production Activity of CdS Nanoflower using Single Atom Pt and Graphene Quantum Dot as Dual Cocatalysts
Yi Yang, Jinsong Wu, Bei Cheng*, Liuyang Zhang*, Ahmed Abdullah Al-Ghamdi, Swelm Wageh and Youji Li
Chin. J. Struct. Chem. 2022, 41, 2206006-2206014 DOI: 10.14102/j.cnki.0254-5861.2022-0124
June 20, 2022
hierarchical nanostructure, single-atom catalysts, graphene quantum dots, CdS nanosheets, hydrogen production
ABSTRACT
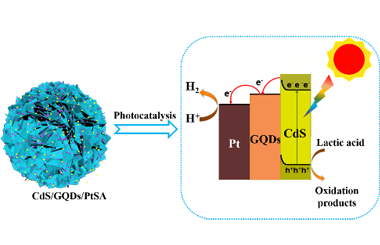
Single-atom catalysts have high catalytic activity due to their unique quantum size effects and optimal atom utilization. Herein, visible-light-responsive photocatalysts were designed by coupling CdS with graphene quantum dots (GQDs) and platinum single atoms (PtSAs). GQDs and PtSAs were successively loaded on ultrathin CdS nanosheets through freeze-drying and in-situ photocatalytic reduction. The synergistic effect between PtSAs and GQDs results in superior photocatalytic activity with a hydrogen production rate of 13488 μmol h-1 g-1 as well as the maximum apparent quantum efficiency (AQE) of 35.5% in lactic acid aqueous solution, which is 62 times higher than that of pristine CdS (213 μmol g-1 h-1). The energy conversion efficiency is ca. 13.05%. As a photosensitizer and an electron reservoir, GQDs can not only extend the light response of CdS to the visible-light region (400-800 nm), but also promotes the separation of photoinduced electron-hole pairs. Meanwhile, PtSAs, with unique electronic and geometric features, can provide more efficient proton reduction sites. This finding provides an effective strategy to remarkably improve photocatalytic H2 production performance. -
S-scheme Porous g-C3N4/Ag2MoO4 Heterojunction Composite for CO2 Photoreduction
Zhongliao Wang, Ruilian Liu, Jinfeng Zhang* and Kai Dai*
Chin. J. Struct. Chem. 2022, 41, 2206015-2206022 DOI: 10.14102/j.cnki.0254-5861.2022-0108
June 20, 2022
S-scheme, g-C3N4, Ag2MoO4, heterojunction, CO2 photoreduction
ABSTRACT
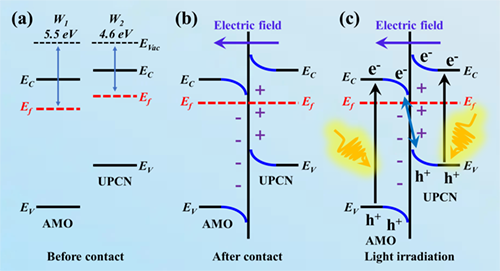
Utilizing solar energy to achieve artificial photosynthesis of chemical fuel is prevalent in tackling excessive CO2 emission and fossil fuel depletion. Grievous charge recombination and weak redox capability aggravate the CO2 photoreduction performance. Engineering tailored morphology and constructing matched heterostructure are two significant schemes to ameliorate the CO2 photoconversion efficiency of g-C3N4-based composite. Herein, a novel S-scheme ultrathin porous g-C3N4 (UPCN)/Ag2MoO4 (AMO) composite was designed by in-situ growing tetragonal α-AMO nanoparticles (NPs) (5-30 nm) on UPCN nanosheets (NSs). The S-scheme charge transfer route endows UPCN/AMO with fast charge separation and strong redox capability, demonstrated by X-ray photoelectron spectroscopy (XPS), photoelectrochemical tests, steady-state and time-resolved photoluminescence (PL) spectra, and DFT calculations. The UPCN/AMO composite exhibits elevated CO2 photoreduction performance with CO and CH4 yield rates of 6.98 and 0.38 μmol g-1 h-1, which are 3.5 and 2.9 folds higher than that of pristine UPCN, respectively. Finally, the CO2 photoreduction intermediates are analyzed, and the CO2 photoreduction mechanism is discussed. This work provides a reference for various g-C3N4-based composites applied in artificial photosynthesis. -
Article
Simultaneous Photocatalytic Oxygen Production and Hexavalent Chromium Reduction in Ag3PO4/C3N4 S-scheme Heterojunction
Tao Yang, Pengke Deng, Lele Wang, Jie Hu*, Qinqin Liu* and Hua Tang*
Chin. J. Struct. Chem. 2022, 41, 2206023-2206030 DOI: 10.14102/j.cnki.0254-5861.2022-0062
June 20, 2022
S-scheme charge transfer route, Ag3PO4/C3N4, Cr(VI) reduction, O2 production, photocatalysis
ABSTRACT
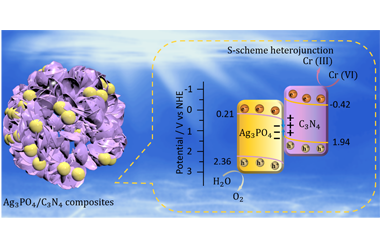
The low separation/migration efficiency is a major obstacle that limits the practical application of semiconductor-photocatalysts. Constructing S-scheme heterojunction is an ideal strategy for providing high photocatalytic activity via accelerating charge separation. Herein, an Ag3PO4/C3N4 composite was synthesized by coupling Ag3PO4 particle with C3N4 hollow spheres in-situ via a precipitation method. The S-scheme heterojunction between Ag3PO4 and C3N4 could accelerate the charge separation and retain high photoredox ability, which synchronously realized high photocatalytic oxygen production and hexavalent chromium reduction. The optimized Ag3PO4/C3N4 composite shows a high oxygen production rate up to 803.31 µmol·g-1·h-1 and a high conversion (87.9%) of Cr(VI) to Cr(III). In addition, C3N4 hollow spheres affords higher reaction efficiency than that of C3N4 tube, C3N4 bulk and C3N4 sheet, which indicates that the hollow sphere structure can provide more active sites and adsorption sites in the photocatalytic process. This work offers an effective way in developing a dual-function S-scheme heterojunction for clean energy production and environmental protection. -
Article
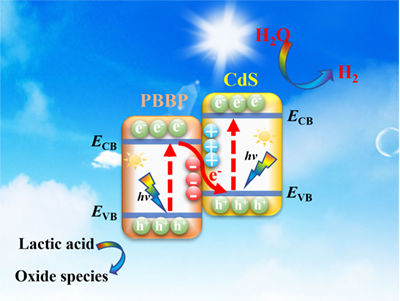
Pyrene-Benzothiadiazole-Based Polymer/CdS 2D/2D Organic/Inorganic Hybrid S-scheme Heterojunction for Efficient Photocatalytic H2 Evolution
Ruiqi Gao, Huan He, Junxian Bai, Lei Hao, Rongchen Shen*, Peng Zhang, Youji Li and Xin Li*
Chin. J. Struct. Chem. 2022, 41, 2206031-2206038 DOI: 10.14102/j.cnki.0254-5861.2022-0096
June 20, 2022
photocatalytic hydrogen evolution, pyrene-benzothiadiazole-based conjugated polymers, S-scheme heterojunction, CdS
ABSTRACT
Nowadays, conjugated polymers have garnered numerous attention as a new class of organic photocatalysts due to their tunable electronic properties, low cost, excellent stability and sufficient light-absorption performance. In particular, pyrene-benzothiadiazole-based conjugated polymer (PBBP) has been considered to be a new type of conjugated polymers for photocatalytic H2 evolution. However, the poor charge separation seriously limits its practical application in H2 evolution. In this work, a PBBP-based polymer/CdS 2D/2D organic/inorganic S-scheme heterojunction photocatalyst with a strong internal electric field is, for the first time, prepared for efficient photocatalytic hydrogen evolution. The pyrene-benzothiadiazole-based conjugated polymers (PBBP) are synthesized by the Suzuki-Miyaura reactions. Then, the hybrid heterojunction photocatalysts are fabricated by coupling CdS with it through the ultrasonic mixing method. As a result, the highest H2-production rate of 15.83 mmol h-1 g-1 is achieved on 20% PBBP/CdS composite under visible-light irradiation, nearly 2.7 times higher than that of pure CdS. The apparent quantum efficiency (AQE) of 20% PBBP/CdS composite could reach 8.66% at λ = 420 nm. The enhanced activity could be attributed to the construction of S-scheme heterojunction, which accelerates the recombination of carriers with weaker redox ability and maintains the strong reducibility of electrons in CdS. This work provides a protocol for pyrene-benzothiadiazole-based conjugated polymers to prepare S-scheme heterojunction photocatalysts based on organic/inorganic coupling. -
Article
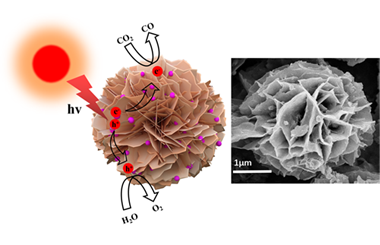
Ag2S Quantum Dots Decorated on Porous Cubic-CdS Nanosheets-Assembled Flowers for Photocatalytic CO2 Reduction
Wei Fu, Jiajie Fan and Quanjun Xiang*
Chin. J. Struct. Chem. 2022, 41, 2206039-2206047 DOI: 10.14102/j.cnki.0254-5861.2022-0090
June 20, 2022
CdS nanosheet, Ag2S quantum dots (QDs), assembled flower structure, composite materials, photocatalytic CO2 reduction
ABSTRACT
The production of renewable fossil fuels such as CH4 and CO by photocatalytic CO2 reduction has attracted more and more attention. However, single photocatalyst is less efficient for photocatalytic reduction of CO2 due to the fast recombination of photogenerated electron pairs. Herein, we successfully prepare CdS-Ag2S composite by assembling the Ag2S QDs cocatalyst on the surface of CdS nanosheet-assembled flower through oil-bath solvothermal method. This composite is prepared through a simple self-assembly strategy using cadmium chloride, ammonia and thiourea as precursors of the CdS nanosheet-assembled flower and silver nitrate and 3-mercaptopropionic acid as the precursors of Ag2S QDs. The average diameter of Ag2S QDs is apparently 6.0 nm. The light absorption edge of the composite is at around 560 nm, with the corresponding band gap at 2.14 eV. The CdS-Ag2S QDs composite with 5 wt% Ag2S QDs loaded achieves CO evolution rate of 16.6 μmol·g-1·h-1 without noble-metal cocatalysts. This strengthened photocatalytic performance and photocatalytic stability were attributed to the energy band broadening of Ag2S QDs caused by quantum size effect and the large specific surface area due to the assembled flower. The mechanism underlying the enhanced photocatalytic CO2 reduction activity is further proposed. This study demonstrates that semiconductor-based quantum dots are strong candidates for excellent cocatalysts in photocatalysis. -
ArticleMolecular Engineering of g-C3N4 with Dibenzothiophene Groups as Electron Donor for Enhanced Photocatalytic H2-Production
Shanren Tao, Sijie Wan, Qinyang Huang, Chengming Li, Jiaguo Yu and Shaowen Cao*
Chin. J. Struct. Chem. 2022, 41, 2206048-2206054 DOI: 10.14102/j.cnki.0254-5861.2022-0068
June 20, 2022
carbon nitride, donor-acceptor, photocatalysis, charge transfer
ABSTRACT
The construction of donor-acceptor (D-A) molecular structure is an attractive strategy to enhance the photocatalytic performance of polymeric semiconductors. Herein, dibenzothiophene (DBT)-4-carbaldehyde as the precursor is introduced into g-C3N4 (TCN) prepared by two-step thermal polymerization to construct an intramolecular D-A type copolymer (TCN-DBTx). DFT calculation and experimental results reveal that DBT plays the role of electron donor unit to modify g-C3N4. The incorporation of DBT not only adjusts the band gap to improve reduction ability, but also induces an internal electric field with extending π-conjugated system for effective charge transfer. As a result, TCN-DBTx exhibits much better photocatalytic performance with an optimal hydrogen production rate of 3334 μmol h-1 g-1, which is 2.5 times that of TCN. This work provides a protocol for preparing high-performance g-C3N4-based photocatalysts toward various applications. -
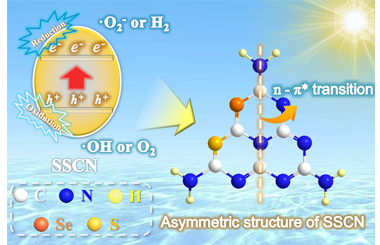
ArticleAsymmetric Structure Awakened n-π* Electron Transition in Sulfur and Selenium Co-doped g-C3N4 with Efficient Photocatalytic Performance
Tian Liu, Yunfeng Li*, Hejia Sun, Min Zhang, Zhiling Xia and Qing Yang
Chin. J. Struct. Chem. 2022, 41, 2206055-2206061 DOI: 10.14102/j.cnki.0254-5861.2022-0152
June 20, 2022
photocatalysis, Co-doping, n-π* transition, charges transfer, photo-degradation
ABSTRACT
Sulfur and selenium co-doped graphitic carbon nitride (SSCN) with efficient photocatalytic activity was synthesized by synchronously introducing sulfur and selenium atoms into the melon structure of g-C3N4 (GCN) via a facile solid-phase thermal reaction of GCN and SeS2. The as-prepared SSCN possesses a larger specific surface area with a richer pore structure that provides more active centers for catalytic reaction. More importantly, the asymmetric structure of SSCN due to introducing sulfur and selenium not only maintains an easier activation of π-π* electron transition but also awakens the n-π* electron transition in g-C3N4. Moreover, the n-π* electron transition of SSCN can be controlled through changing the amount of SeS2, which can greatly extend the photo-response range to 600 nm. As a result, the SSCN samples show an excellent photo-degradation performance for typical antibiotic of tetracycline hydrochloride (TC). The specific degradation route and main intermediates of TC based on liquid chromatograph mass spectrometer (LC-MS) analysis are also investigated and discussed. -
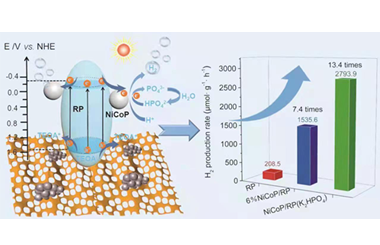
ArticleK2HPO4-Mediated Photocatalytic H2 Production over NiCoP/RP Heterojunction
Junfeng Huang, Chenyang Li, Xiaoyun Hu, Jun Fan, Binran Zhao* and Enzhou Liu*
Chin. J. Struct. Chem. 2022, 41, 2206062-2206068 DOI: 10.14102/j.cnki.0254-5861.2021-0055
June 20, 2022
photocatalysis, hydrogen, red phosphorus, bimetallic cocatalyst, K2HPO4
ABSTRACT
In this work, bimetallic NiCoP nanoparticles (NPs) were firstly prepared by a solvothermal method using red phosphorus (RP) as P source, and it was combined with RP nanosheets via a physical grinding process. Investigation indicates that NiCoP has better charge transfer ability and faster H2 releasing kinetics than the corresponding single metal phosphides alone. 6 wt% NiCoP/RP exhibits an excellent H2 evolution activity in 20 vol.% triethanol-amine/water solution under a 300W Xe-lamp irradiation, and the corresponding H2 production rate is 1535.6 μmol·g-1·h-1, which is 7.4, 3.2 and 2.6 times higher than those of pure RP, 6 wt% Co2P/RP and 6 wt% Ni2P/RP, respectively. In addition, we demonstrate that K2HPO4 can further enhance the H2 evolution kinetics by inducing a new H+ reduction path, when appropriate K2HPO4 is introduced into the reaction solution. The H2 production rate of 6 wt% NiCoP/RP is boosted from 1535.6 to 2793.9 μmol·g-1·h-1 due to the easier combination between H+ and electrons with the assistance of HPO42-. It is 13.4 times higher than that of pure RP. This work demonstrates that bimetallic phosphides with suitable electrolytes can greatly enhance the photocatalytic H2 evolution efficiency. -
Article
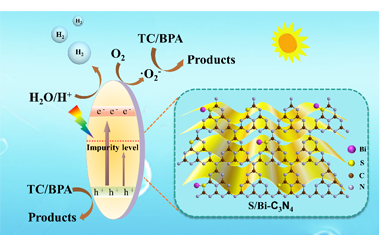
Bi and S Co-doping g-C3N4 to Enhance Internal Electric Field for Robust Photocatalytic Degradation and H2 Production
Yan Hu, Xibao Li*, Weiwei Wang, Fang Deng*, Lu Han*, Xiaoming Gao, Zhijun Feng, Zhi Chen, Juntong Huang, Fanyan Zeng and Fan Dong
Chin. J. Struct. Chem. 2022, 41, 2206069-2206078 DOI: 10.14102/j.cnki.0254-5861.2022-0103
June 20, 2022
photocatalysis, g-C3N4, co-doping, IEF strength, antibiotic
ABSTRACT
By adjusting the type and proportion of doping elements in the g-C3N4-based photocatalyst, the internal electric field (IEF) strength of the semiconductor can be regulated. This can effectively enhance the driving force of charge separation in the photocatalytic process. It is found that the introduction of appropriate concentration of Bi and S into the skeleton structure of g-C3N4 can achieve efficient degradation of tetracycline (TC) and other pollutants in the liquid environment and excellent photocatalytic H2 evolution performance (1139 μmol·L-1·h-1). Since the prepared samples have similar crystal structures, the relative strength of IEF can be calculated. It can be used as the basis for adjusting the IEF strength of g-C3N4-based semiconductor by element doping. In addition, the Bi and S co-doped g-C3N4 samples after solvothermal reflux show good chemical stability and can reduce the nanostructure defects caused by co-doping of heteroatoms, thus it provides a novel solution for the construction of g-C3N4-based dual-function photocatalyst with high activity and stability. -
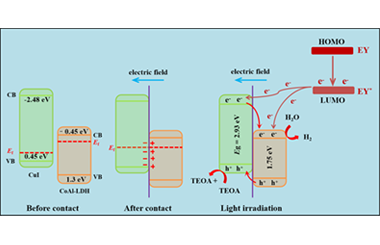
ArticleIngenious Design of CoAl-LDH p-n Heterojunction Based on CuI as Holes Receptor for Photocatalytic Hydrogen Evolution
Yue Cao, Hongqian Gou, Pengfei Zhu and Zhiliang Jin*
Chin. J. Struct. Chem. 2022, 41, 2206079-2206085 DOI: 10.14102/j.cnki.0254-5861.2022-0042
June 20, 2022
CoAl-LDH, CuI, p-n heterojunction, photocatalysis, hydrogen production
ABSTRACT
Reasonable design of heterojunction can greatly improve the photocatalytic hydrogen evolution activity of materials. Herein, p-n heterojunction of 2D/3D structure is constructed by the nanosheet of CoAl-LDH and rock-like CuI. The introduction of CuI can make CoAl-LDH disperse better, which brings more reaction sites for the hydrogen evolution reaction. Meanwhile, the 2D/3D structure is conducive to the construction of p-n heterojunction between the CoAl-LDH and CuI. The optical and electrochemical properties of the material indicate that the separation and transference of photon-generated carriers are promoted by the p-n heterojunction. The activity of composite catalyst (CI-10) reaches a maximum of 3.59 mmol g-1h-1 which is 28.5 times higher than that of CuI. Furthermore, the influence of the amount of CuI and pH value on the hydrogen evolution reaction is explored. Based on the band structures of CoAl-LDH and CuI, the mechanism of photocatalytic reaction of CI-10 is proposed. The p-n heterojunction constructed with the CuI as hole receptor provides a new way to enhance the activity of photocatalytic H2 evolution.