Ruiqi Gao, Huan He, Junxian Bai, Lei
Hao,
Rongchen Shen*, Peng Zhang, Youji Li and Xin Li*
The blue cover, together with elements such as blisters, lightning and the sun, highlights the good prospect of this new type of heterojunction as a photocatalytic material. The "S" shaped dragon depicted by the element of water rises from the bottom, implying that the dragon gets water and magically turns water into green hydrogen over organic/inorganic S-type heterojunction photocatalyst under sunlight irradiation.
K2HPO4-Mediated
Photocatalytic H2 Production over NiCoP/RP Heterojunction
Junfeng Huang, Chenyang Li, Xiaoyun Hu, Jun Fan, Binran Zhao* and Enzhou Liu*
Chin. J. Struct. Chem. 2022, 41, 2206062-2206068 DOI: 10.14102/j.cnki.0254-5861.2021-0055
June 20, 2022
photocatalysis, hydrogen, red phosphorus, bimetallic cocatalyst, K2HPO4
ABSTRACT
In
this work, bimetallic NiCoP nanoparticles (NPs) were firstly prepared by a solvothermal
method using red phosphorus (RP) as P source, and it was combined with RP
nanosheets via a physical grinding process. Investigation indicates that
NiCoP has better charge transfer ability and faster H2 releasing kinetics than the corresponding single metal phosphides
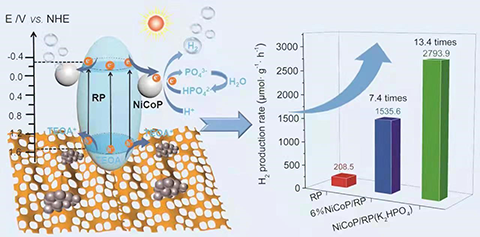
alone. 6 wt% NiCoP/RP exhibits an excellent H2 evolution
activity in 20 vol.% triethanol-amine/water solution under a 300W
Xe-lamp irradiation, and the corresponding H2 production rate is
1535.6 μmol·g-1·h-1, which is 7.4, 3.2 and 2.6 times
higher than those of pure RP, 6 wt% Co2P/RP and 6 wt%
Ni2P/RP, respectively. In addition, we demonstrate that K2HPO4 can further enhance the H2 evolution kinetics by inducing a new H+ reduction path, when appropriate K2HPO4 is introduced into the
reaction solution. The H2 production rate of 6 wt% NiCoP/RP is boosted from 1535.6 to 2793.9 μmol·g-1·h-1 due to the easier combination between H+ and electrons with the
assistance of HPO42-. It is 13.4 times higher than that
of pure RP. This work demonstrates that bimetallic phosphides with suitable
electrolytes can greatly enhance the photocatalytic H2 evolution efficiency.