Ruiqi Gao, Huan He, Junxian Bai, Lei
Hao,
Rongchen Shen*, Peng Zhang, Youji Li and Xin Li*
The blue cover, together with elements such as blisters, lightning and the sun, highlights the good prospect of this new type of heterojunction as a photocatalytic material. The "S" shaped dragon depicted by the element of water rises from the bottom, implying that the dragon gets water and magically turns water into green hydrogen over organic/inorganic S-type heterojunction photocatalyst under sunlight irradiation.
Ingenious Design of CoAl-LDH p-n Heterojunction
Based on CuI as Holes Receptor for Photocatalytic Hydrogen Evolution
Yue Cao, Hongqian Gou, Pengfei Zhu and Zhiliang Jin*
Chin. J. Struct. Chem. 2022, 41, 2206079-2206085 DOI: 10.14102/j.cnki.0254-5861.2022-0042
June 20, 2022
CoAl-LDH, CuI, p-n heterojunction, photocatalysis, hydrogen production
ABSTRACT
Reasonable design of heterojunction can greatly improve the photocatalytic hydrogen evolution
activity of materials. Herein, p-n heterojunction of 2D/3D
structure is constructed by the nanosheet of CoAl-LDH and rock-like CuI. The introduction of CuI can make CoAl-LDH disperse better,
which brings more reaction sites for the hydrogen evolution reaction. Meanwhile, the 2D/3D structure is
conducive to the construction of p-n heterojunction between the CoAl-LDH and
CuI. The optical and electrochemical properties of the material indicate that
the separation and transference of photon-generated carriers are promoted by
the p-n heterojunction. The activity of composite
catalyst (CI-10) reaches a maximum of 3.59 mmol g-1h-1 which is 28.5 times higher
than that of CuI. Furthermore, the influence of the amount of CuI and pH value
on the hydrogen evolution reaction is explored. Based on the band structures of
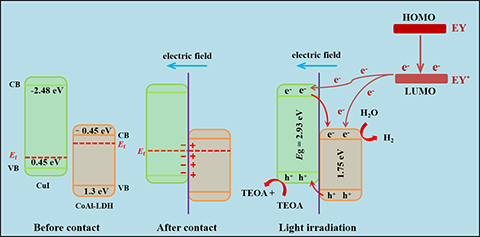
CoAl-LDH and CuI, the mechanism of photocatalytic reaction of CI-10 is
proposed. The p-n heterojunction constructed with the CuI as hole receptor
provides a new way to enhance the activity of photocatalytic H2 evolution.