CeO2 Particles Anchored to Ni2P Nanoplate for Efficient Photocatalytic Hydrogen Evolution
Teng Yan, Xiaojie Zhang, Hua Liu* and Zhiliang Jin*
Chin. J. Struct. Chem. 2022, 41, 2201047-2201053 DOI: 10.14102/j.cnki.0254-5861.2021-0057
January 13, 2022
CeO2, Ni2P, photocatalysis, hydrogen evolution
ABSTRACT
Photocatalytic
hydrogen evolution can convert intermittent and dispersive solar energy into
hydrogen with high energy density, which is expected to fundamentally solve the
problems of environmental pollution and energy shortages. In this experiment,
the performance of the catalyst is modified by introducing cocatalyst and
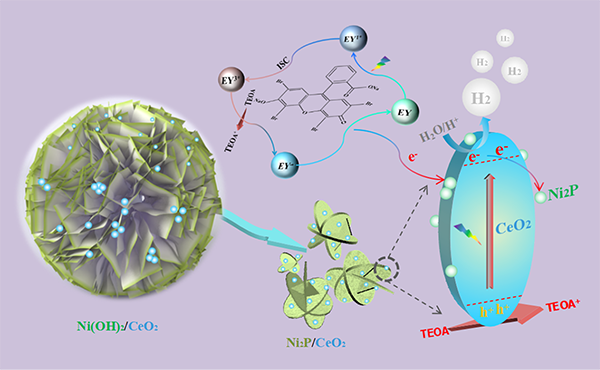
morphology control. Ni(OH)2 nanoflowers are used as substrates to
derive nanoplate stack Ni2P by high-temperature phosphating method,
and a great many of CeO2 nanoparticles are anchored in the Ni2P.
This unique 3D/0D combination effectively inhibits the agglomeration of CeO2 nanoparticles and shortens the electron transfer path. Secondly, the
introduction of metal-like performance of Ni2P broadens the light
absorption range of the catalyst and reduces the overpotential of the catalyst,
which is a key factor in enhancing the catalytic activity. The design ideas of
this experiment have reference significance for the design of efficient and
environmentally friendly photocatalysts.