Cover Picture
Insight into stable, concentrated radicals from sulfur-functionalized alkyne-rich crystalline frameworks and application in solar-to-vapor conversion
Jian-Rong Li, Jieying Hu , Lai-Hon Chung, Jilong Zhou, Parijat Borah, Zhiqing Lin, Yuan-Hui Zhong, Hua-Qun Zhou, Xianghua Yang, Zhengtao Xu*, Jun He*
Submit a Manuscript
Insight into stable, concentrated radicals from sulfur-functionalized alkyne-rich crystalline frameworks and application in solar-to-vapor conversion
Jian-Rong Li, Jieying Hu , Lai-Hon Chung, Jilong Zhou, Parijat Borah, Zhiqing Lin, Yuan-Hui Zhong, Hua-Qun Zhou, Xianghua Yang, Zhengtao Xu*, Jun He*
Submit a Manuscript
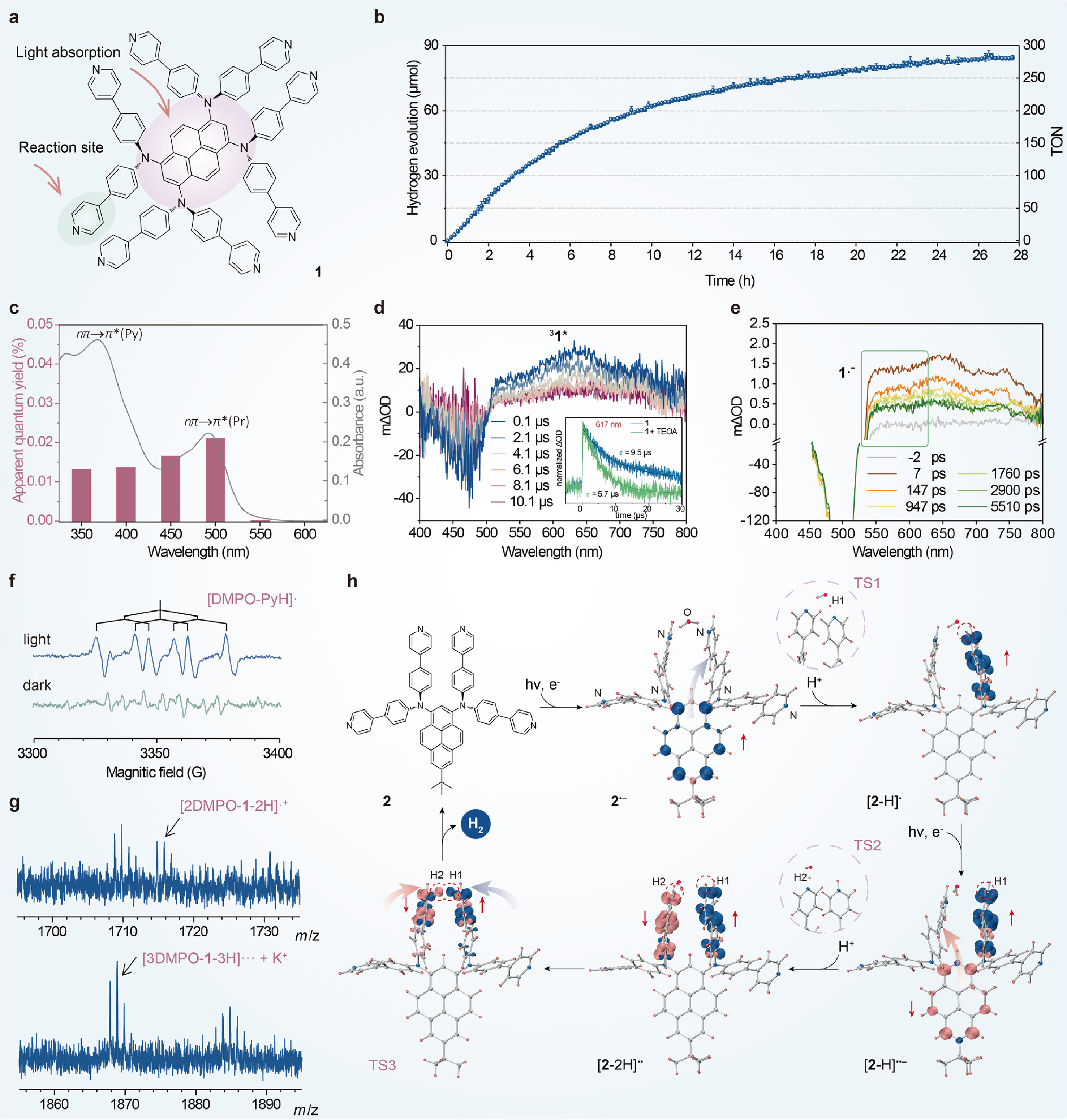
Water reduction by an organic single-chromophore photocatalyst
Kun Tang, Yu-Wu Zhong*
Chin. J. Struct. Chem., 2024, 43: 100376. DOI: 10.1016/j.cjsc.2024.100376
August 15, 2024
ABSTRACT
Up to date, the most common
reactive intermediate for water reduction is metal hydride. The PyH• intermediate proposed and manifested in this work offers a new perspective
for the mechanism understanding of known photocatalytic systems and the design
of new photocatalysts. The PyH• intermediate is generated from the pyridine reaction centers via the
light-induced PCET process, which is directly involved for the subsequent H–H
bond formation via the hemolytic elimination of two PyH• units. Recent theoretical investigations suggest that pyridinic nitrogen
atoms of π-conjugated polymers may also act as the hydrogen-adsorbing sites for
hydrogen evolution. It is of great interest to investigate that whether similar
heterocycle-hydrogen radical intermediate is generated and involved in the
water reduction catalyzed by carbon nitride and COF materials. The
clarification of the reaction site and mechanism of these photocatalysts will
be of great insignificance for the development of next-generation simpler,
greener, and low-cost photocatalysts.