A new SIFSIX anion pillared cage MOF with crs topological structure for efficient C2H2/CO2 separation
Huirong Chen, Yingzhi He, Yan Han, Jianbo Hu, Jiantang Li, Yunjia Jiang, Basem Keshta, Lingyao Wang, Yuanbin Zhang* Submit a Manuscript
Yutong Xiong, Ting Meng, Wendi Luo, Bin Tu, Shuai Wang, Qingdao Zeng*
Chin. J. Struct. Chem., 2025, 44(2), 100511. DOI: 10.1016/j.cjsc.2025.100511
February 1, 2025
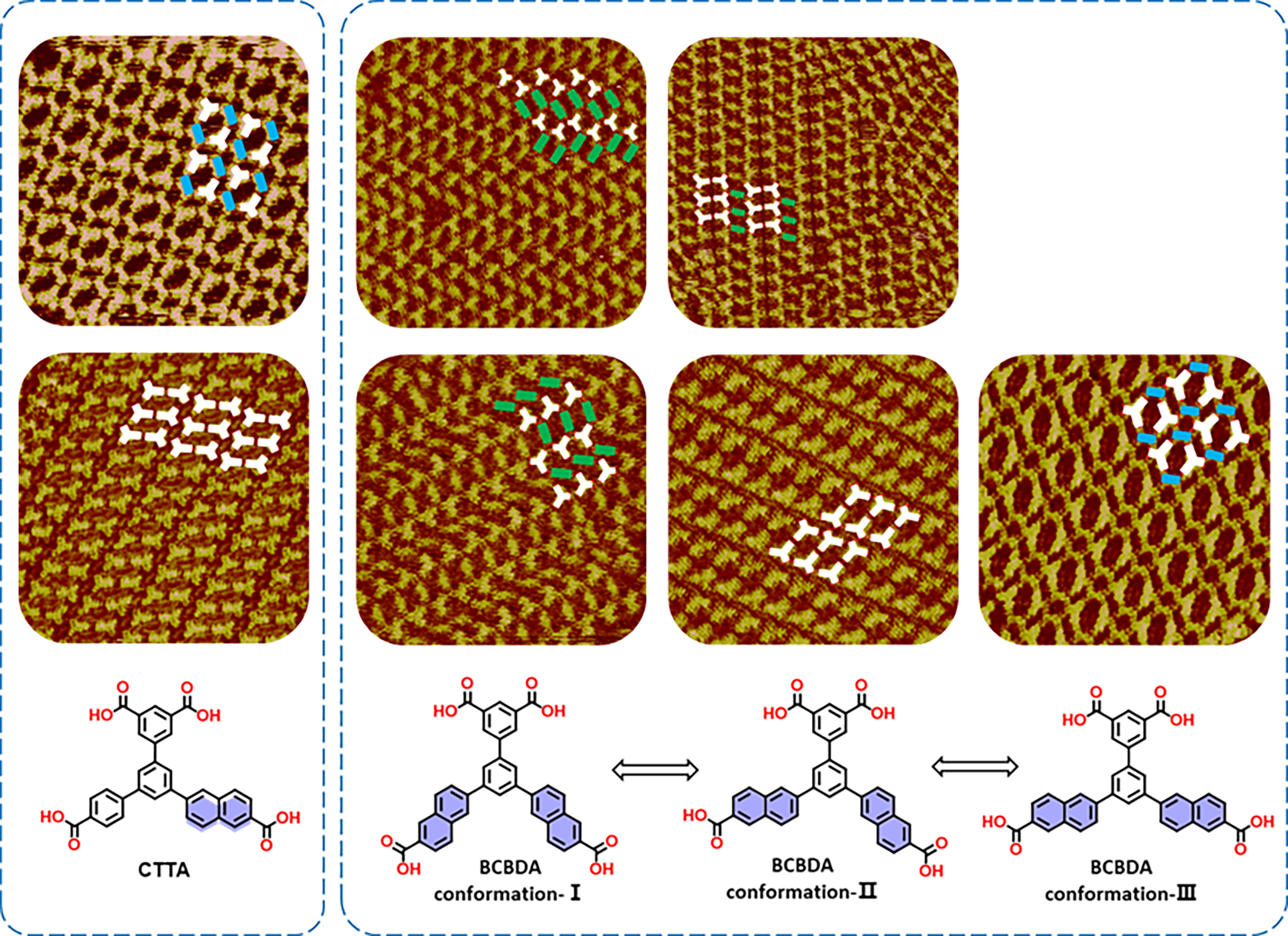
Co-assembly; Conformation; Hydrogen bonds; Scanning tunneling microscopy; DFT calculations
ABSTRACT
The assembly behaviors of two low-symmetric carboxylic acid molecules (5′-(6-carboxynaphthalen-2-yl)-[1,1′:3′,1′′-triphenyl]-3,4′′,5-tricarboxylic acid (CTTA) and 3′,5′-bis(6-carboxynaphthalen-2-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid (BCBDA)) containing naphthalene rings on graphite surfaces have been investigated using scanning tunneling microscopy (STM). The transformation of nanostructures induced by the second components (EDA and PEBP-C4) have been also examined. Both CTTA and BCBDA molecules self-assemble at the 1-heptanoic acid (HA)/HOPG interface, forming porous network structures. The dimer represents the most elementary building unit due to the formation of double hydrogen bonds. Moreover, the flipping of naphthalene ring results in the isomerization of BCBDA molecule. The introduction of carboxylic acid derivative EDA disrupts the dimer, which subsequently undergoes a structural conformation to form a novel porous structure. Furthermore, upon the addition of pyridine derivative PEBP-C4, N–H⋯O hydrogen bonds are the dominant forces driving the three co-assembled structures. We have also conducted density functional theory (DFT) calculations to determine the molecular conformation and analyze the mechanisms underlying the formation of nanostructures.