Cover Picture
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
High thermal conductivity in Ga2TeO6 crystals: Synergistic effects of rigid polyhedral frameworks and stereochemically inert cations
Ziyi Liu, Feifei Guo*, Tingting Cao, Youxuan Sun, Xutang Tao, Zeliang Gao* Submit a Manuscript
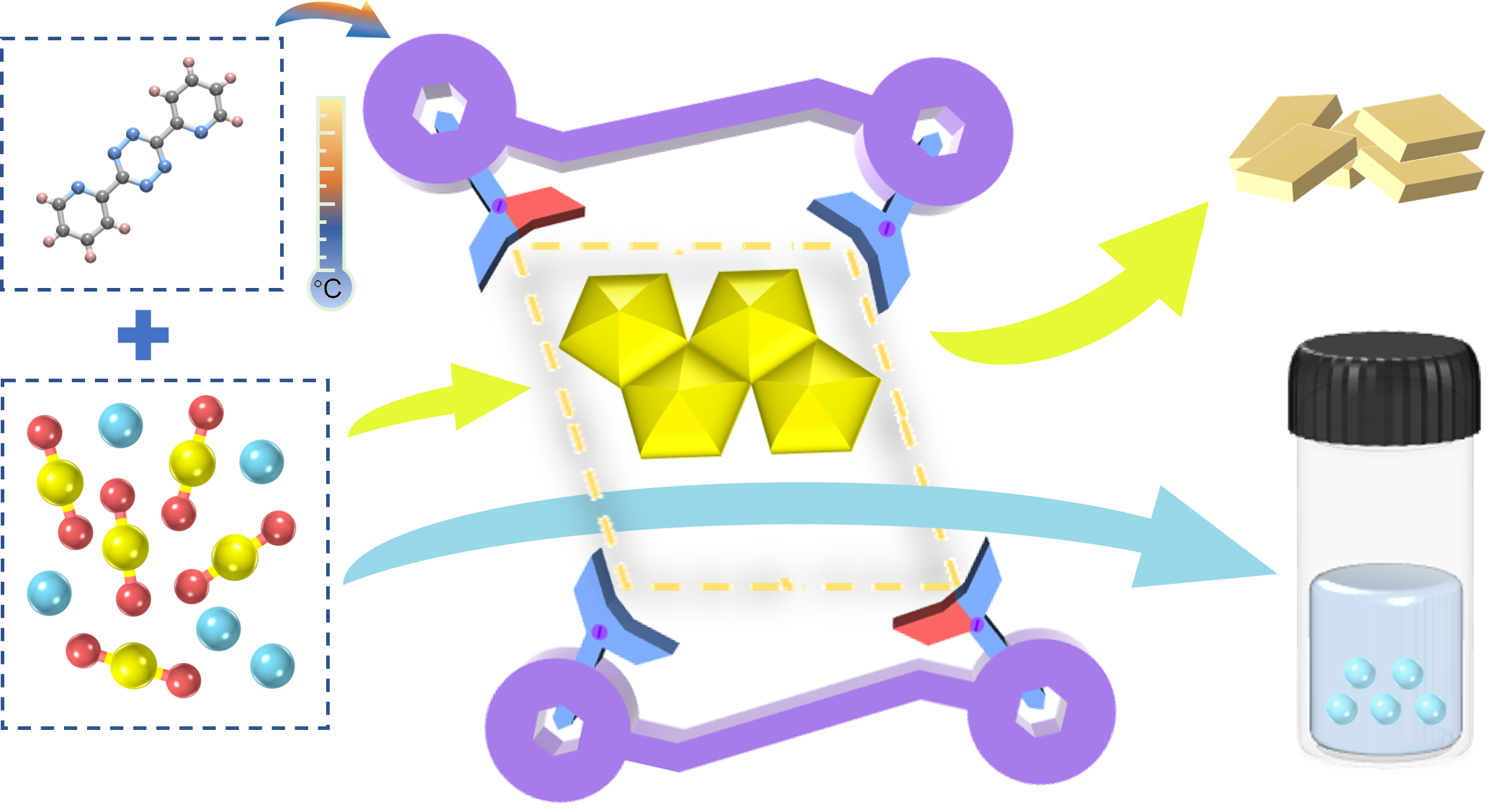
Highly efficient separation of high-valent actinide ions from lanthanides via fractional crystallization
Yarui Li, Huangjie Lu, Yingzhe Du, Jie Qiu*, Peng Lin*, Jian Lin*
Chin. J. Struct. Chem., 2025, 44(4), 100562. DOI: 10.1016/j.cjsc.2025.100562
April 1, 2025
Lanthanide; Actinide; Uranium; Separation; Crystallization
ABSTRACT
Partitioning of actinides from lanthanides is pivotal for advancing nuclear waste management and sustaining nuclear energy development, yet it remains a formidable challenge due to the intricate chemical behaviors of these f-block elements. In this study, we introduce 3,6-di-2-pyridyl-1,2,4,5-tetrazine (L1), whose hydrolysis product of pyridine-2-carbox-aldehyde (pyridine-2-carbonyl)-hydrazone (L2) can fractionally crystallize U(VI) ions over Ln(III) cations with high selectivity and efficiency. Through hydrolysis-induced C–N bond cleavage, L2 acts as a tetradentate ligand, coordinating with two UO22+ ions in a planar arrangement to form a zero-dimensional cluster, [(UO2)2(μ3-O)(L2)(CH3COO)]·DMF (U-L2), while lanthanide ions (Ln = La, Pr, Nd, Sm, Eu, Gd, Tb, Yb, and Lu) remain in solution due to their inability to achieve similar coordination. This selective crystallization strategy yields exceptional separation factors (SFs) between U(VI) and Ln(III), with a value of 756276 between U(VI) and Sm(III), the highest reported to date. Furthermore, this fractional crystallization separation process can be achieved under mild ambient conditions with high SFs, enabling the development of a rapid, safe and energy-efficient strategy for once-through separation of high oxidation state actinides from lanthanides.